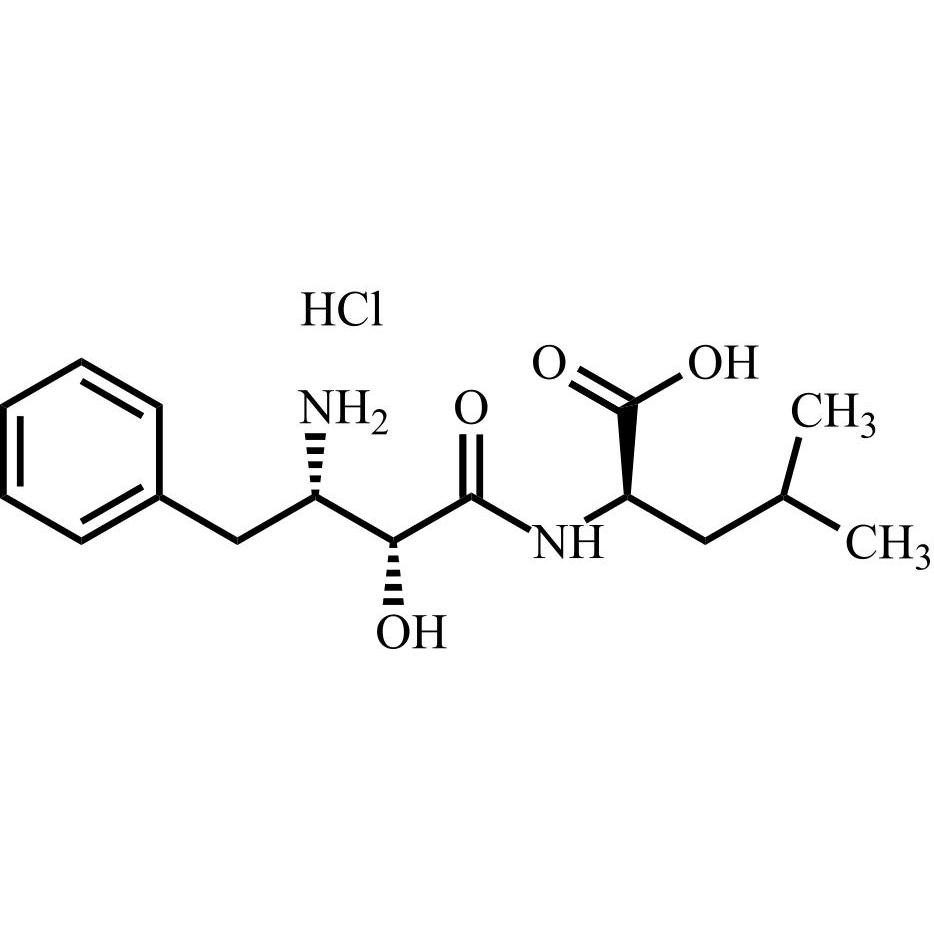
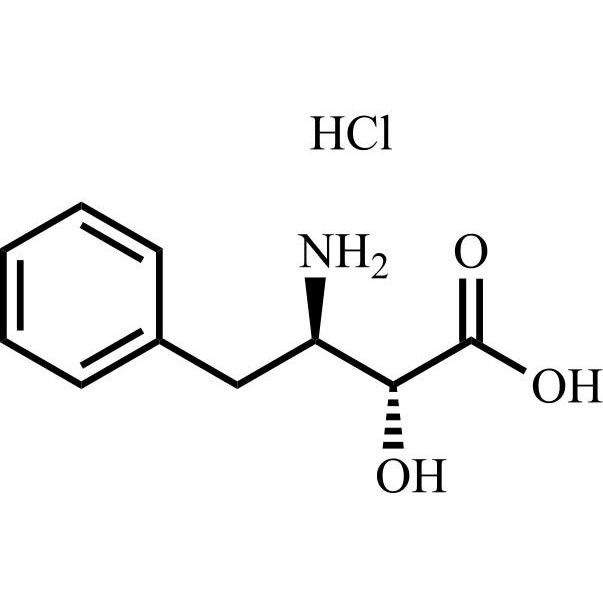
Bestatin Impurity 17 HCl is a fully characterized chemical compound used as a reference standard of API Bestatin. The standard offered is compliant with regulatory guidelines. Bestatin Impurity 17 HCl is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA
Bestatin Impurity 17 HCl is a fully characterized chemical compound used as a reference standard of API Bestatin. The standard offered is compliant with regulatory guidelines. Bestatin Impurity 17 HCl is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA