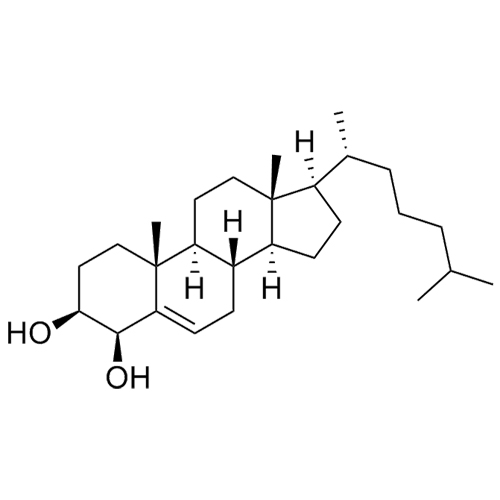
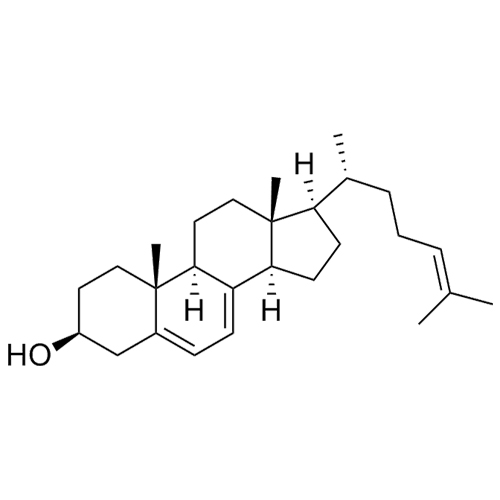
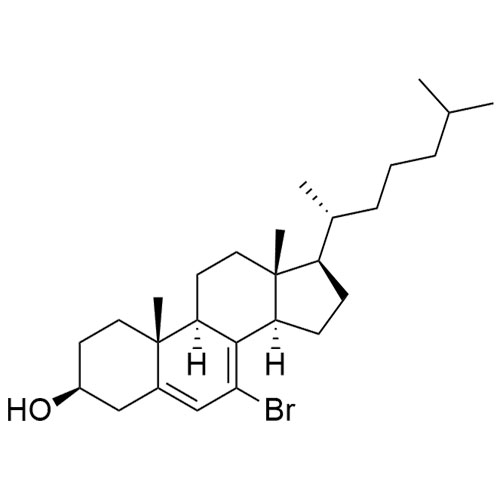
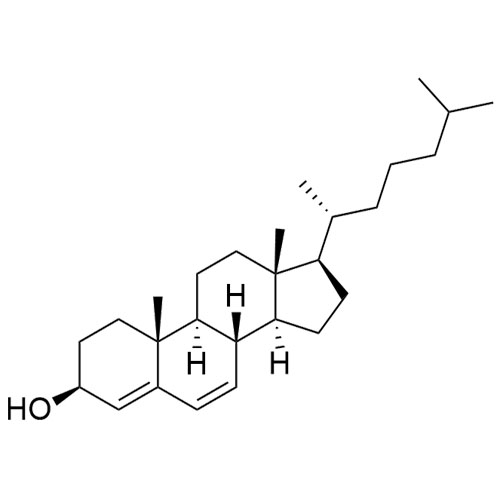
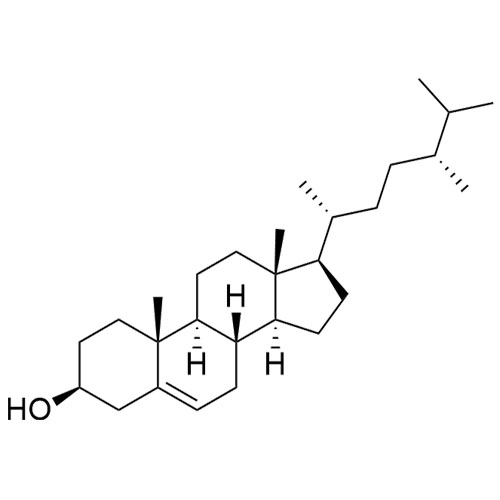
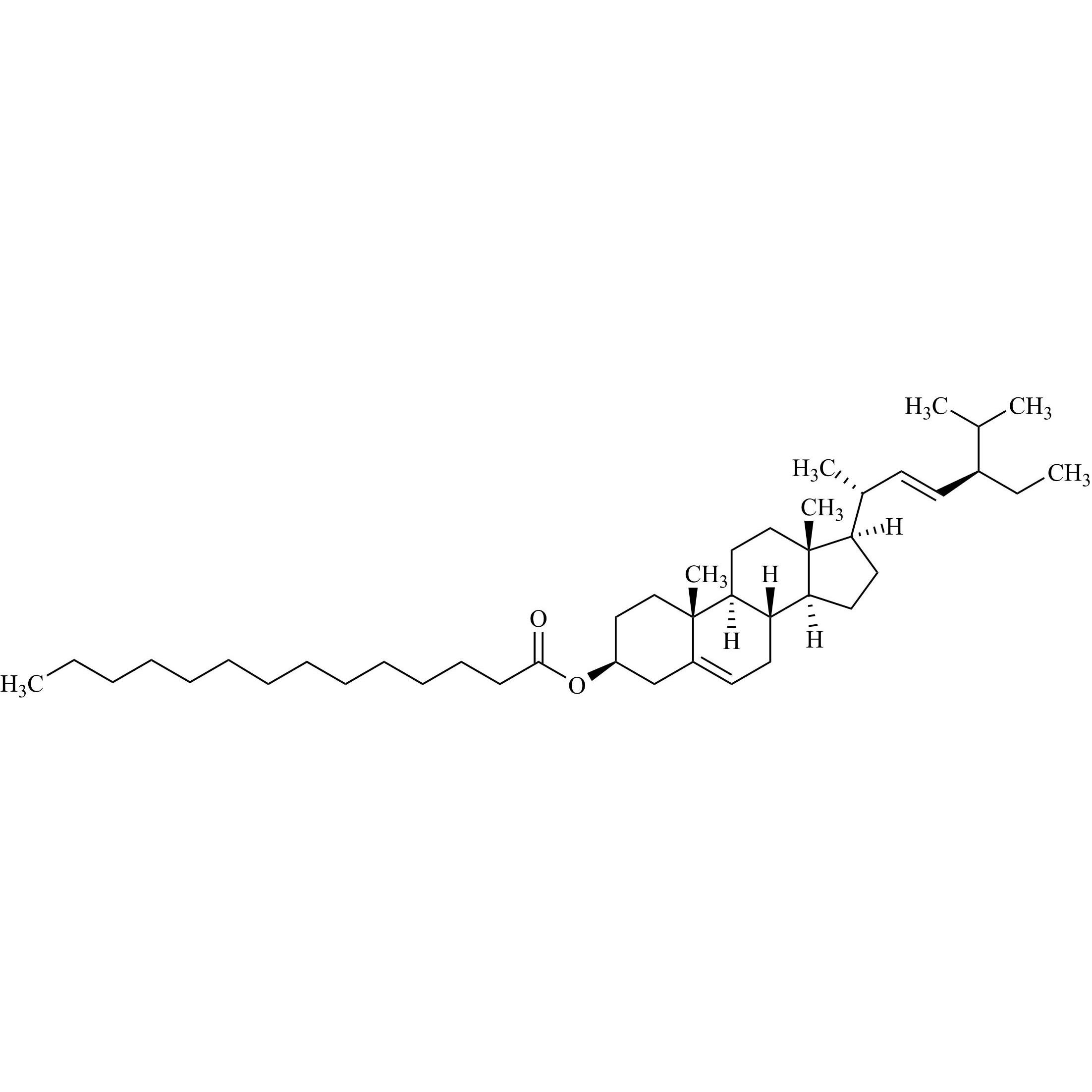
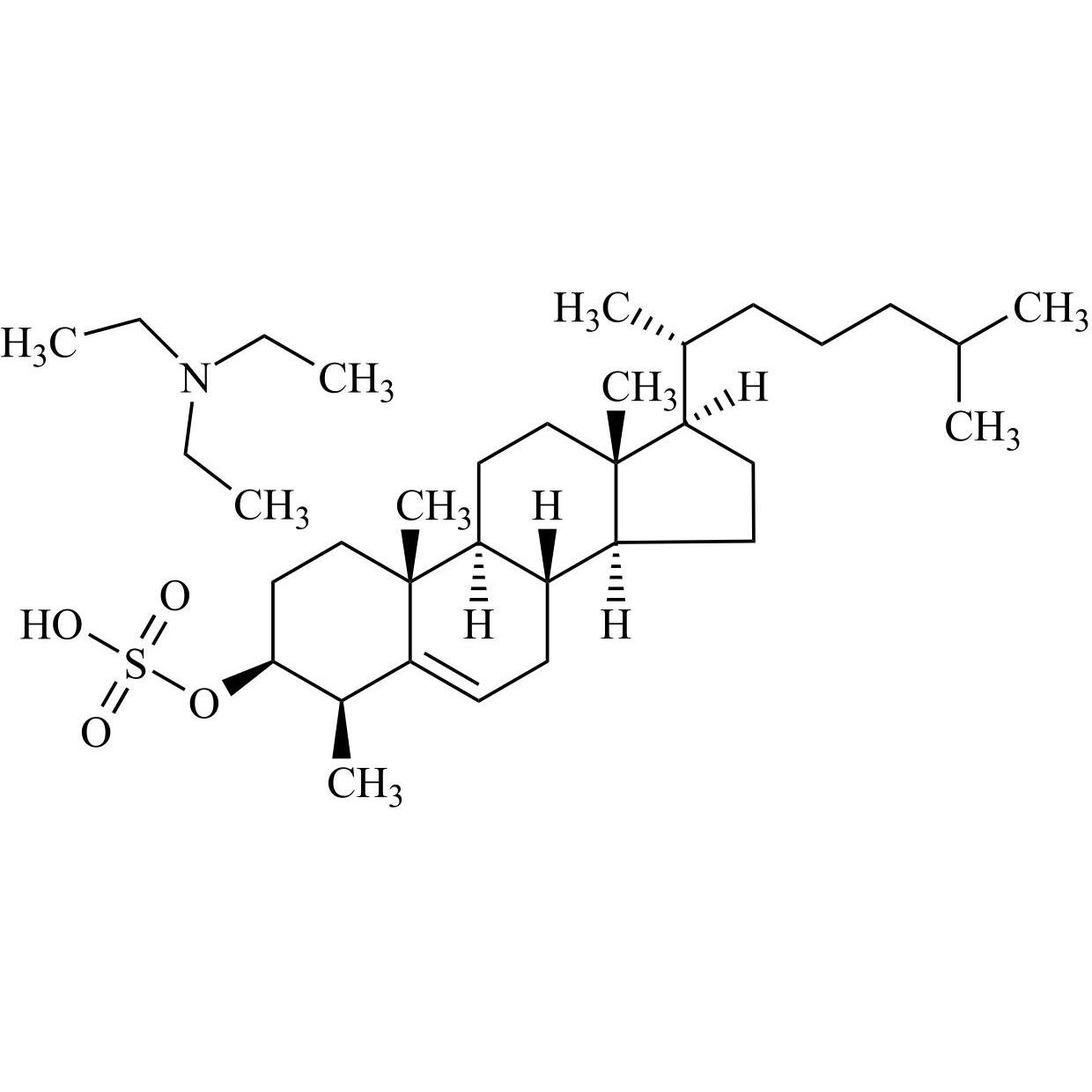
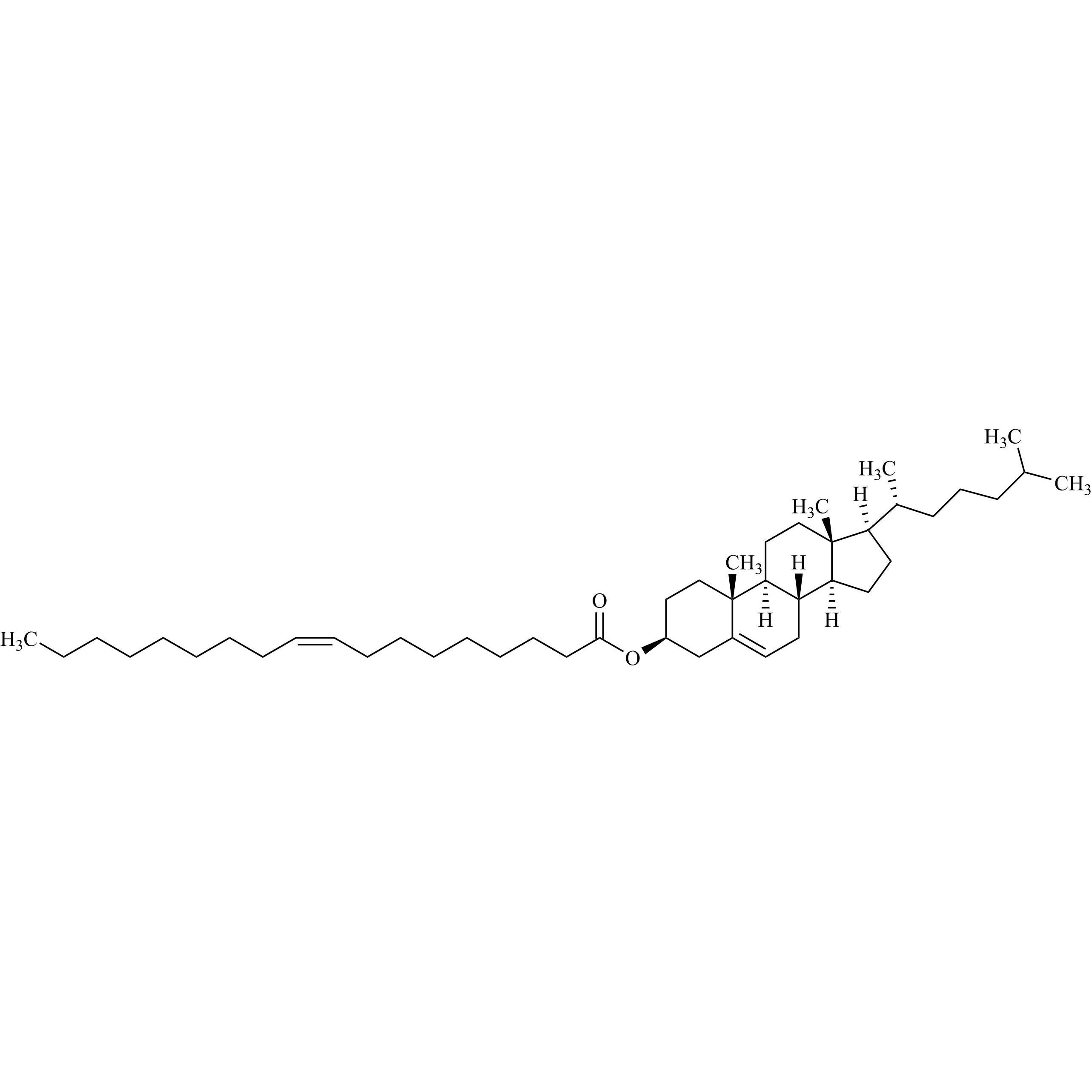
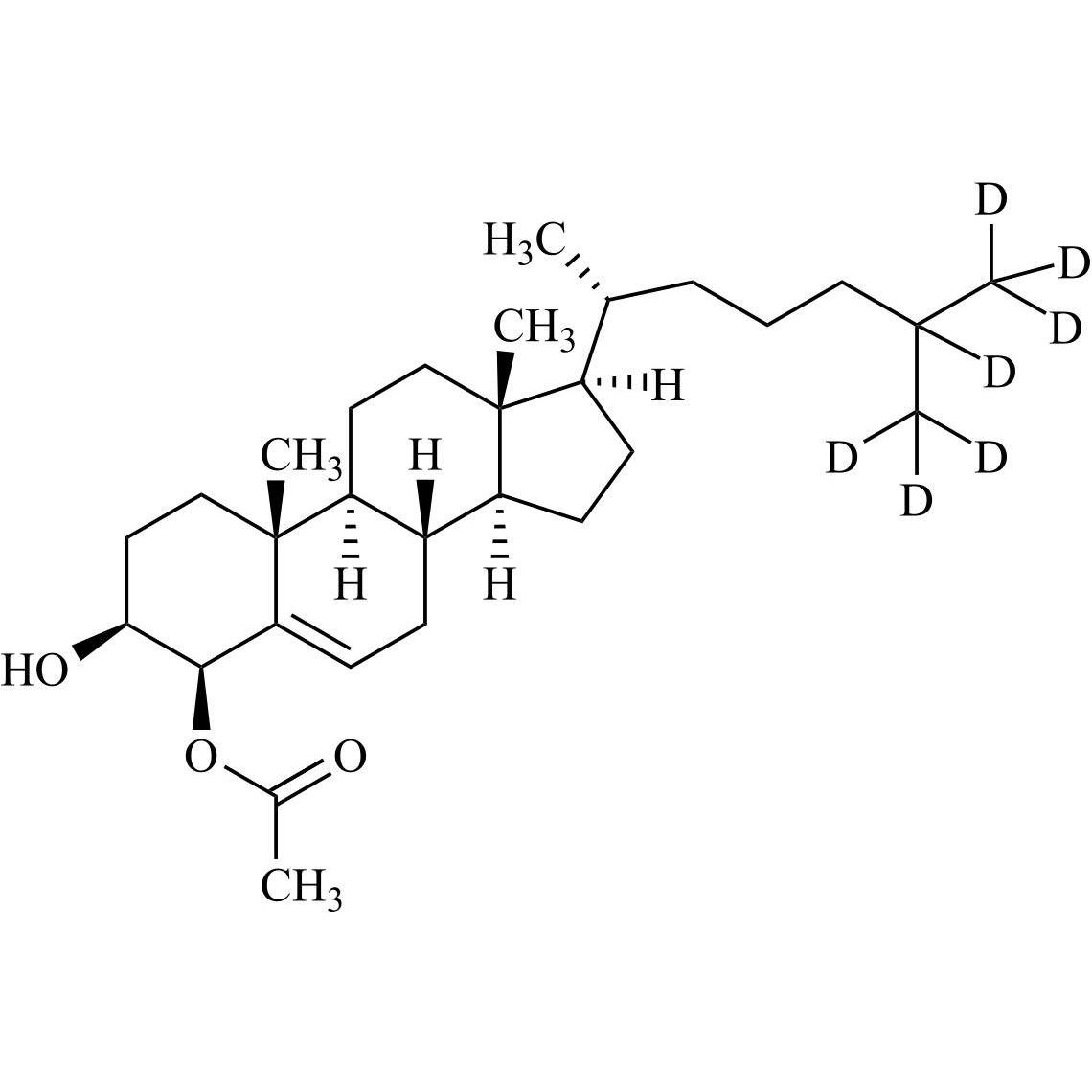
Cholesterol Impurity 33 is a fully characterized chemical compound used as a reference standard of API Cholesterol. The standard offered is compliant with regulatory guidelines. Cholesterol Impurity 33 is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA
Related products
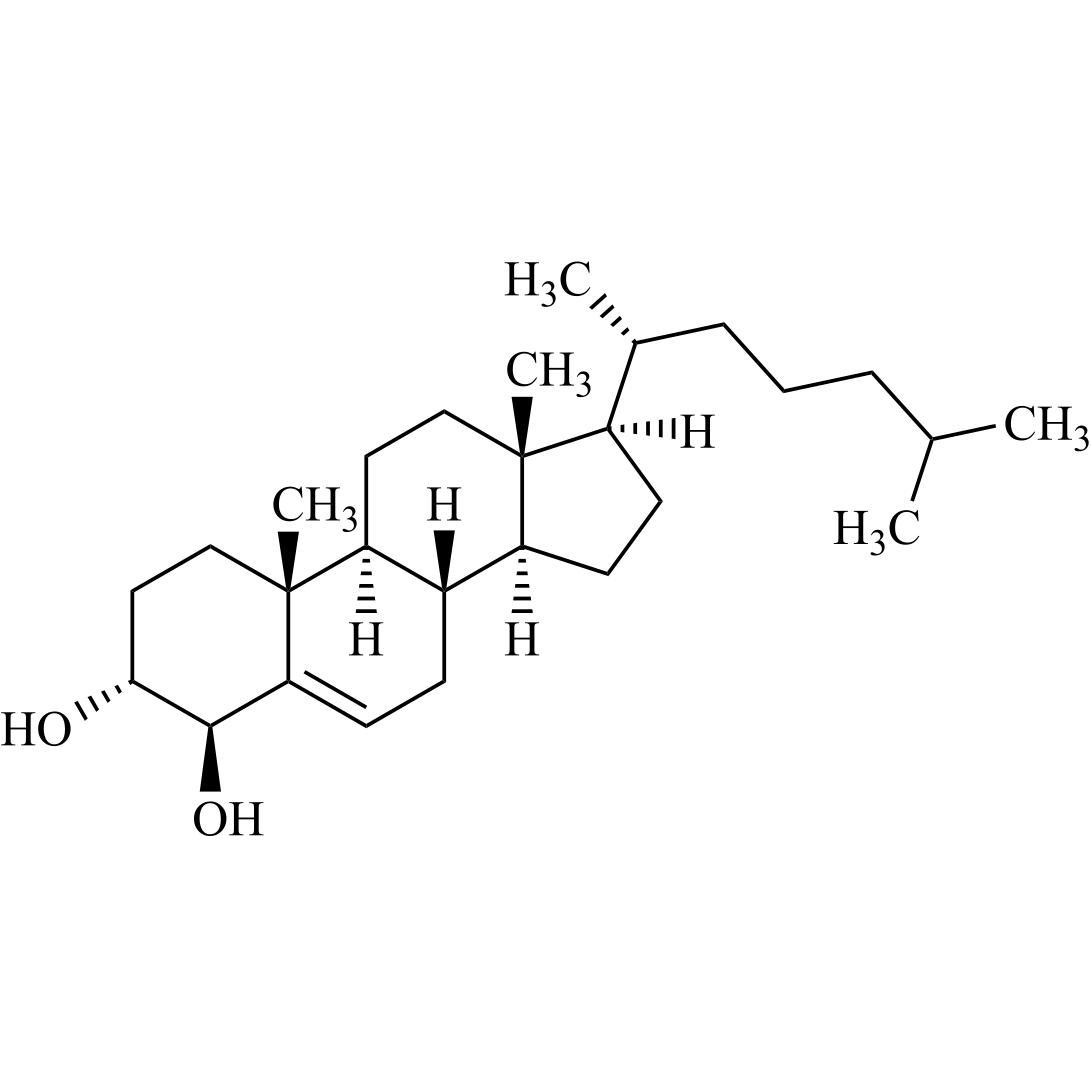
Cholecalciferol EP Impurity B (3-beta-7-Dehydro Cholesterol (Cholesta-5,7-dien-3-beta-ol))

M.F.
M.W. 384.65
CAT# AR-C02599
CAS# 434-16-2
Cholesterol Impurity 7 Triethylamine Salt (Desmosterol Sulfate Triethylamine Salt)

M.F.
M.W. 464.71 101.19
CAT# AR-C07330
CAS# NA
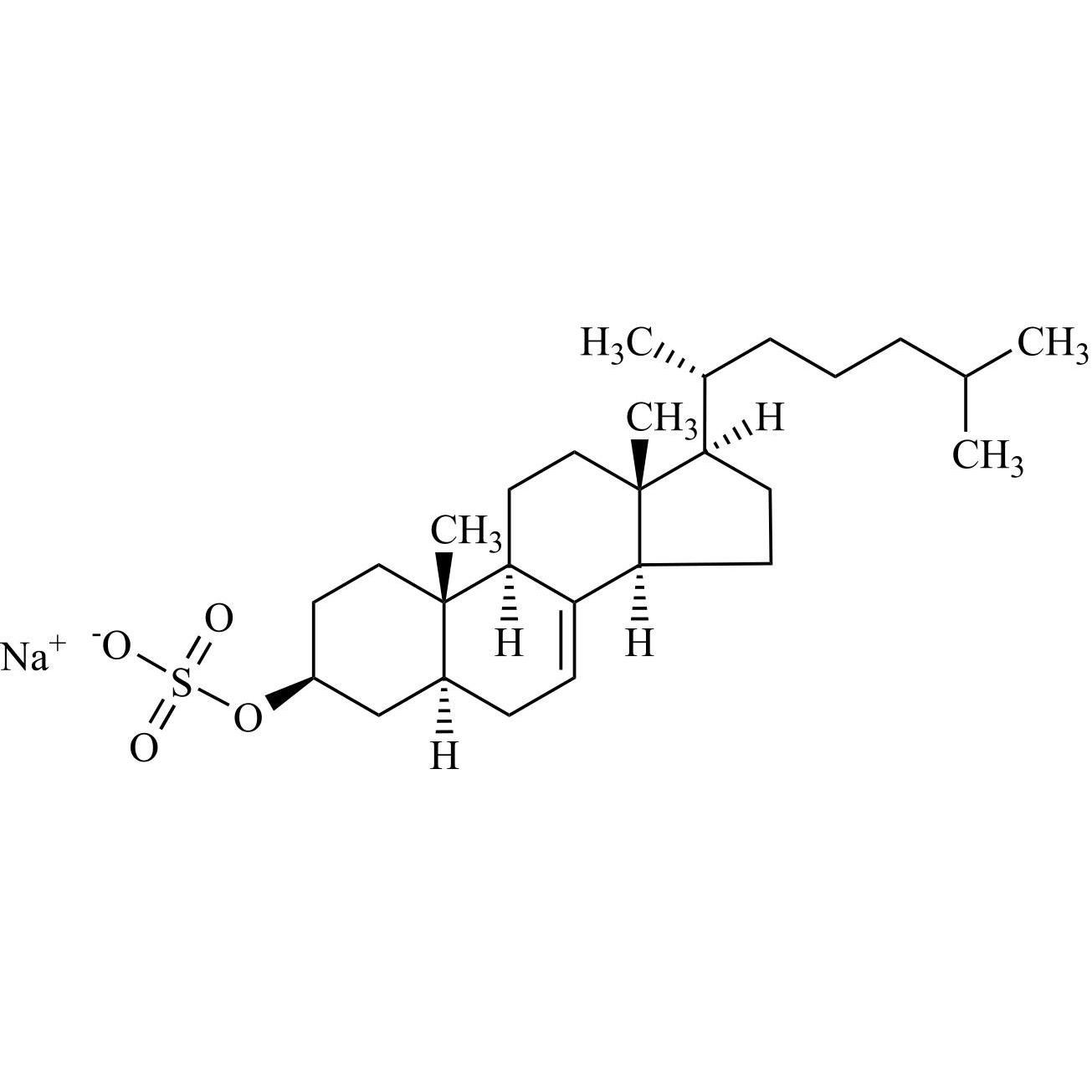
Cholesterol Sulfate Triethylamine Salt

M.F.
M.W. 466.72 101.19
CAT# AR-C07329
CAS# 1256-86-6 (free acid)
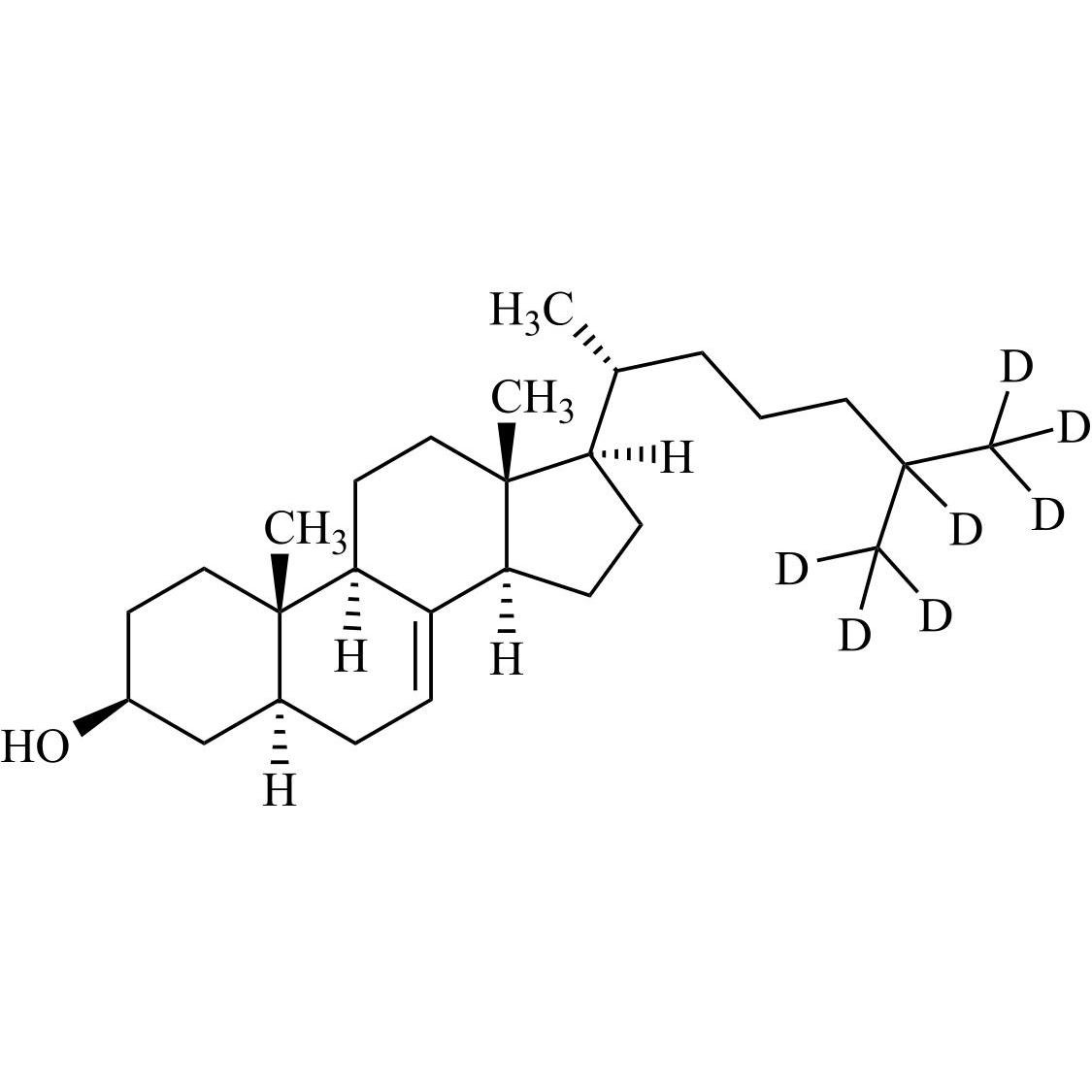
Cholecalciferol EP Impurity B-d7 (3-beta-7-Dehydro Cholesterol-d7)
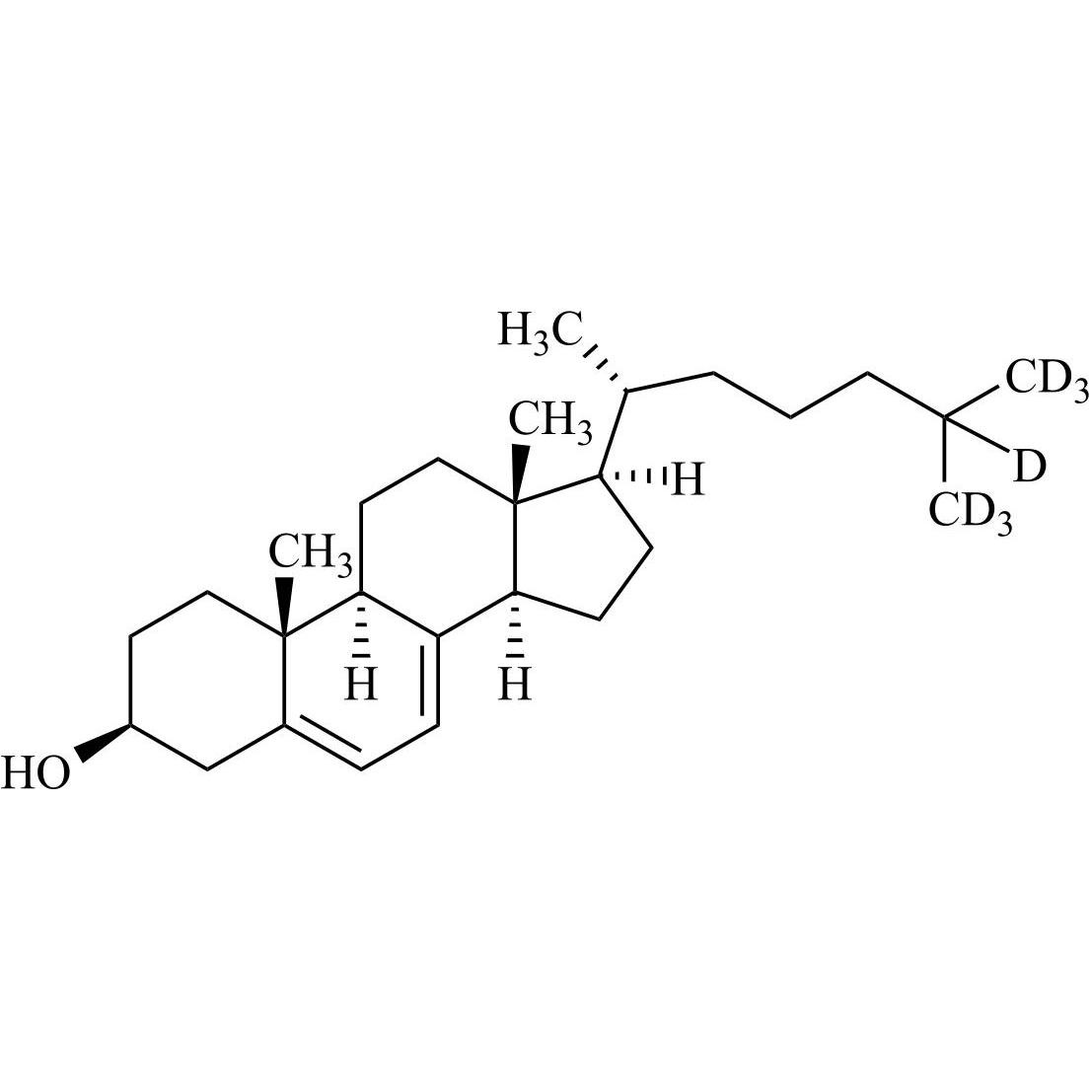
M.F.
M.W. 391.69
CAT# AR-C07344
CAS# 388622-58-0