Piperacillin EP Impurity B (Mixture of Diastereomers)

M.F. C₂₃H₂₉N₅O₈S
M.W. 535.57
CAT# AR-P02032
CAS# NA

M.F. C₂₃H₂₉N₅O₈S
M.W. 535.57
CAT# AR-P02032
CAS# NA

M.F. C₂₅H₃₁N₅O₉S
M.W. 577.184
CAT# AR-P29640
CAS# 1706671-50-2
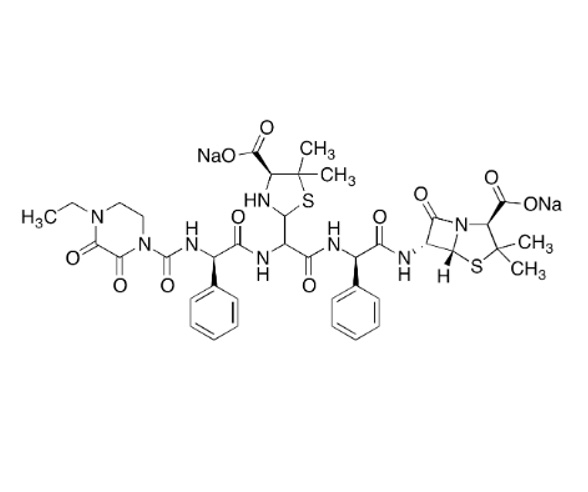
M.F. C₃₉H₄₄N₈Na₂O₁₁S₂
M.W. 910.92
CAT# AR-P29734
CAS# NA

M.F. C₂₅H₃₁N₅O₉S
M.W. 577.61
CAT# AR-P29830
CAS# NA

M.F. C₁₇H₂₀N₄O₆
M.W. 376.37
CAT# AR-P02056
CAS# 2170771-47-6

M.F. C₄₆H₅₄N₁₀O₁₄S₂
M.W. 1035.11
CAT# AR-P29433
CAS# 2170771-53-4
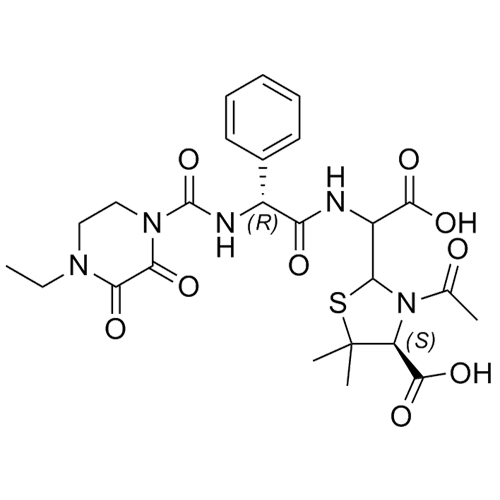
M.F. C₂₅H₃₁N₅O₉S
M.W. 577.62
CAT# AR-P02036
CAS# NA
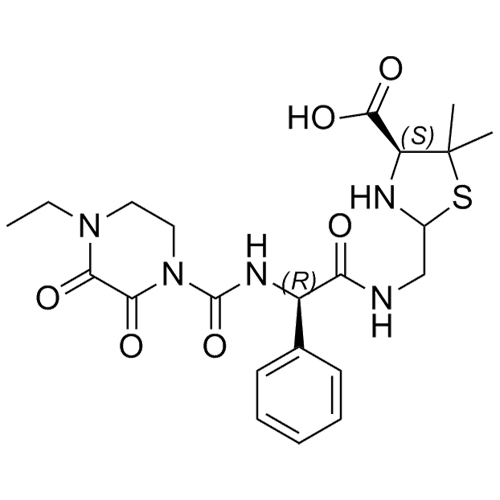
M.F. C₂₂H₂₉N₅O₆S
M.W. 491.56
CAT# AR-P02033
CAS# 2649505-15-5
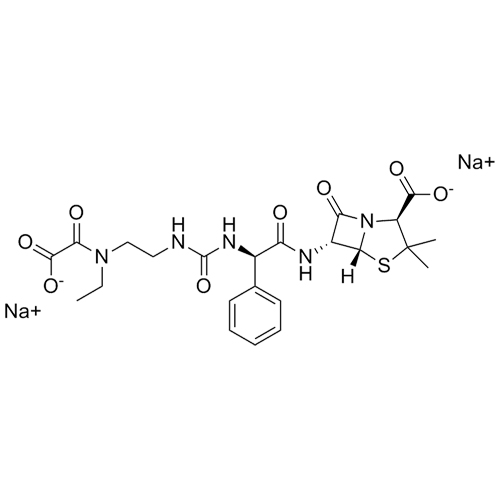
M.F. C₂₃H₂₉N₅O₈S; ₂Na
M.W. 533.56 2 22.99
CAT# AR-P02058
CAS# NA

M.F. C₂₃H₂₉N₅O₈S
M.W. 535.57
CAT# AR-P29536
CAS# 1449784-97-7
Can't find what you are looking for?
Inquire Now