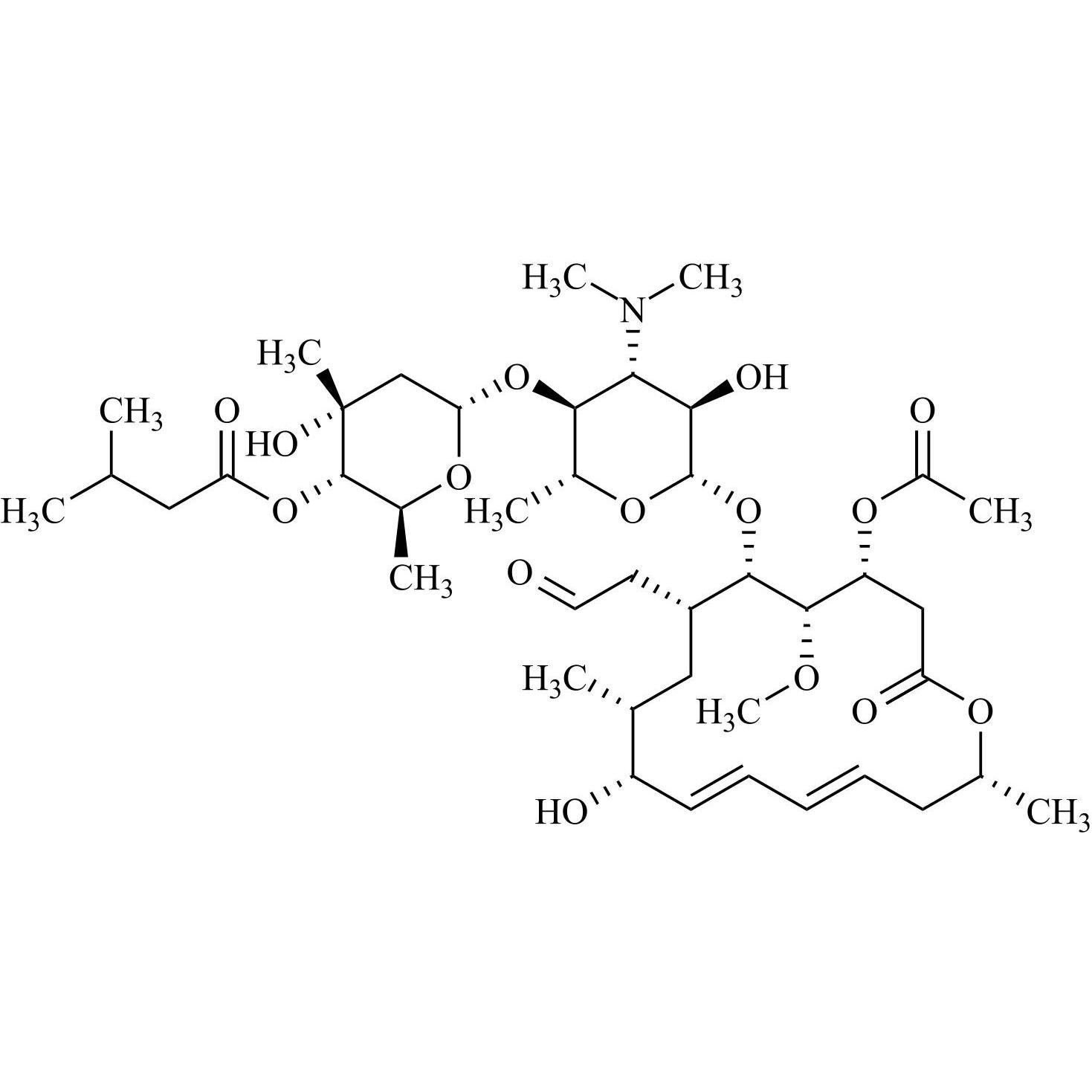
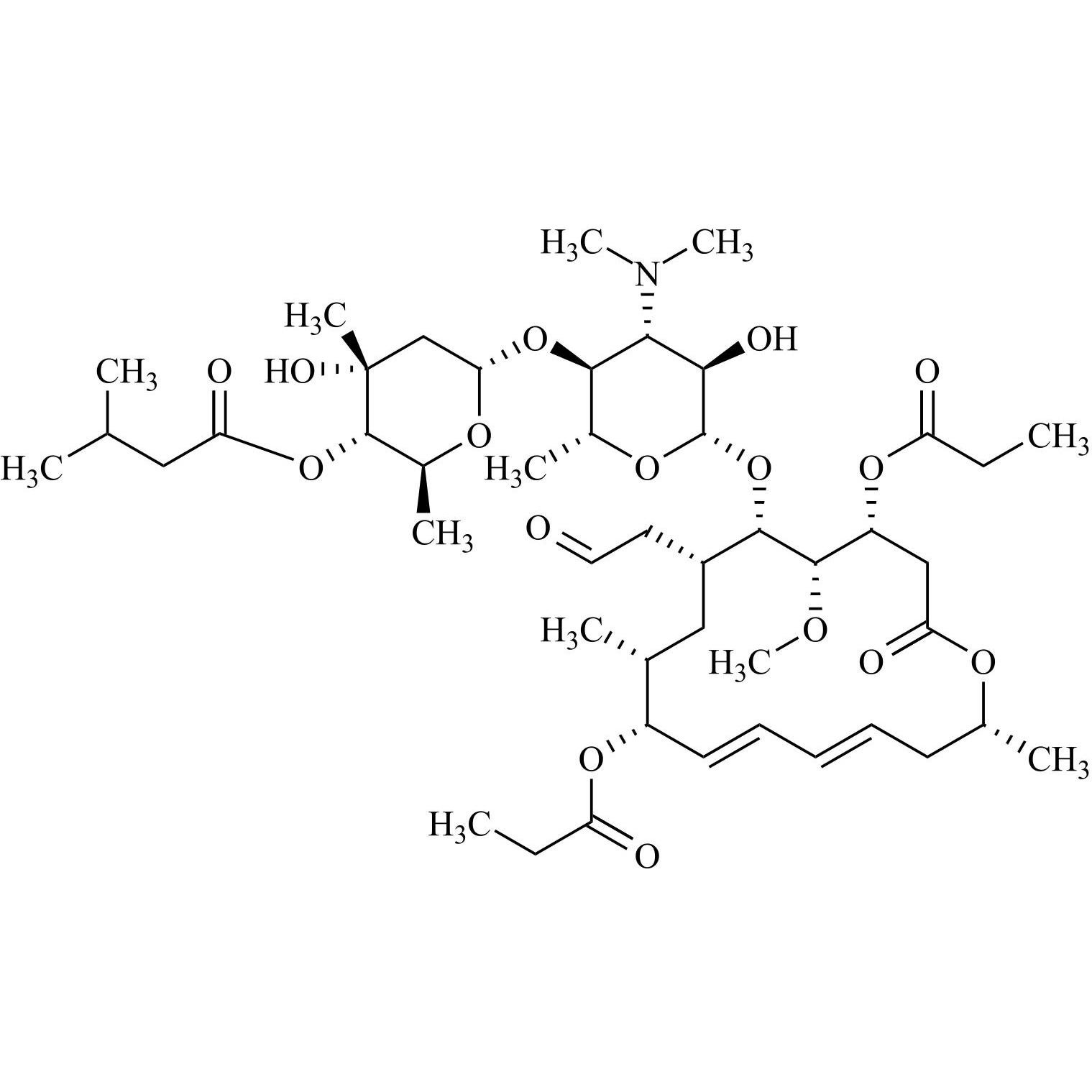
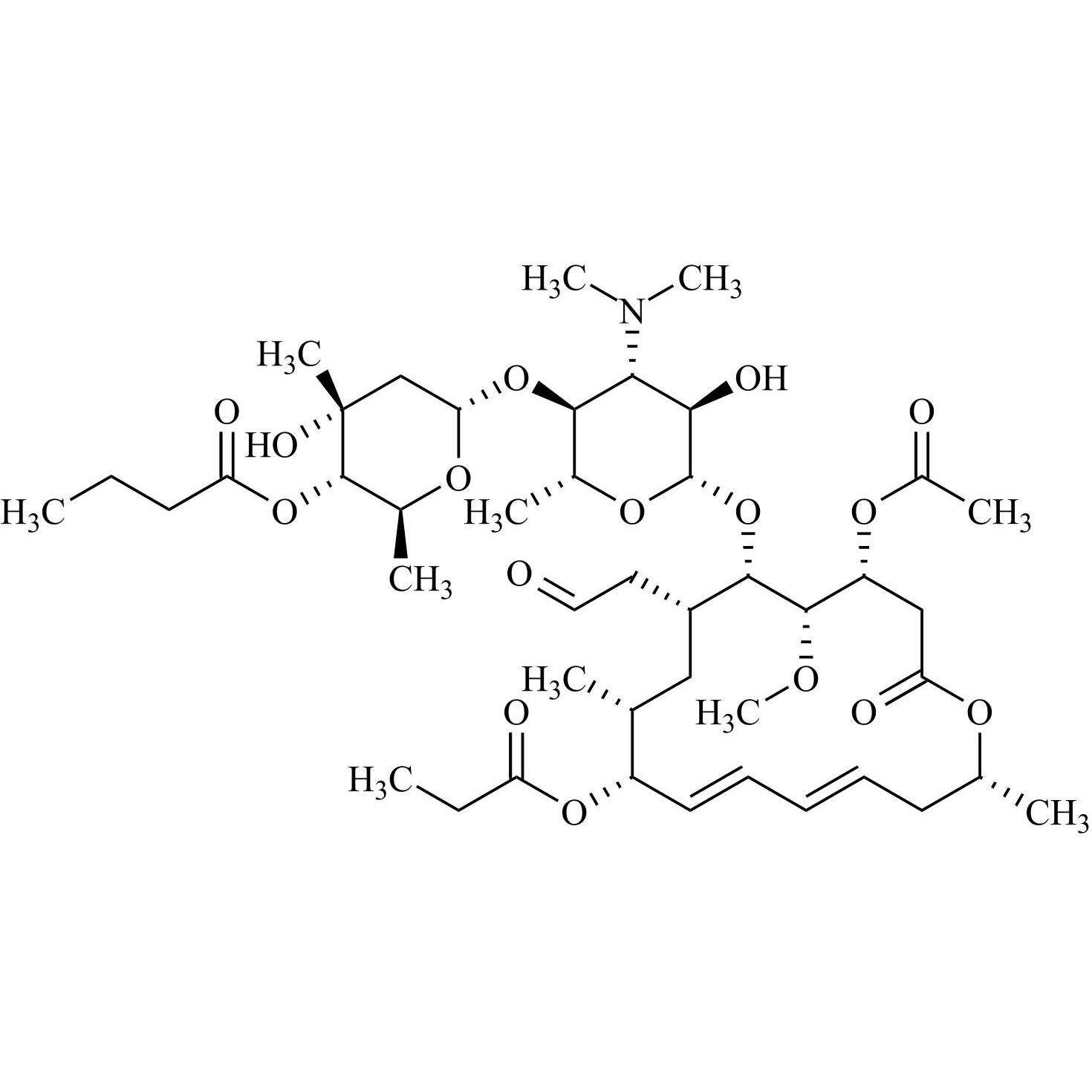
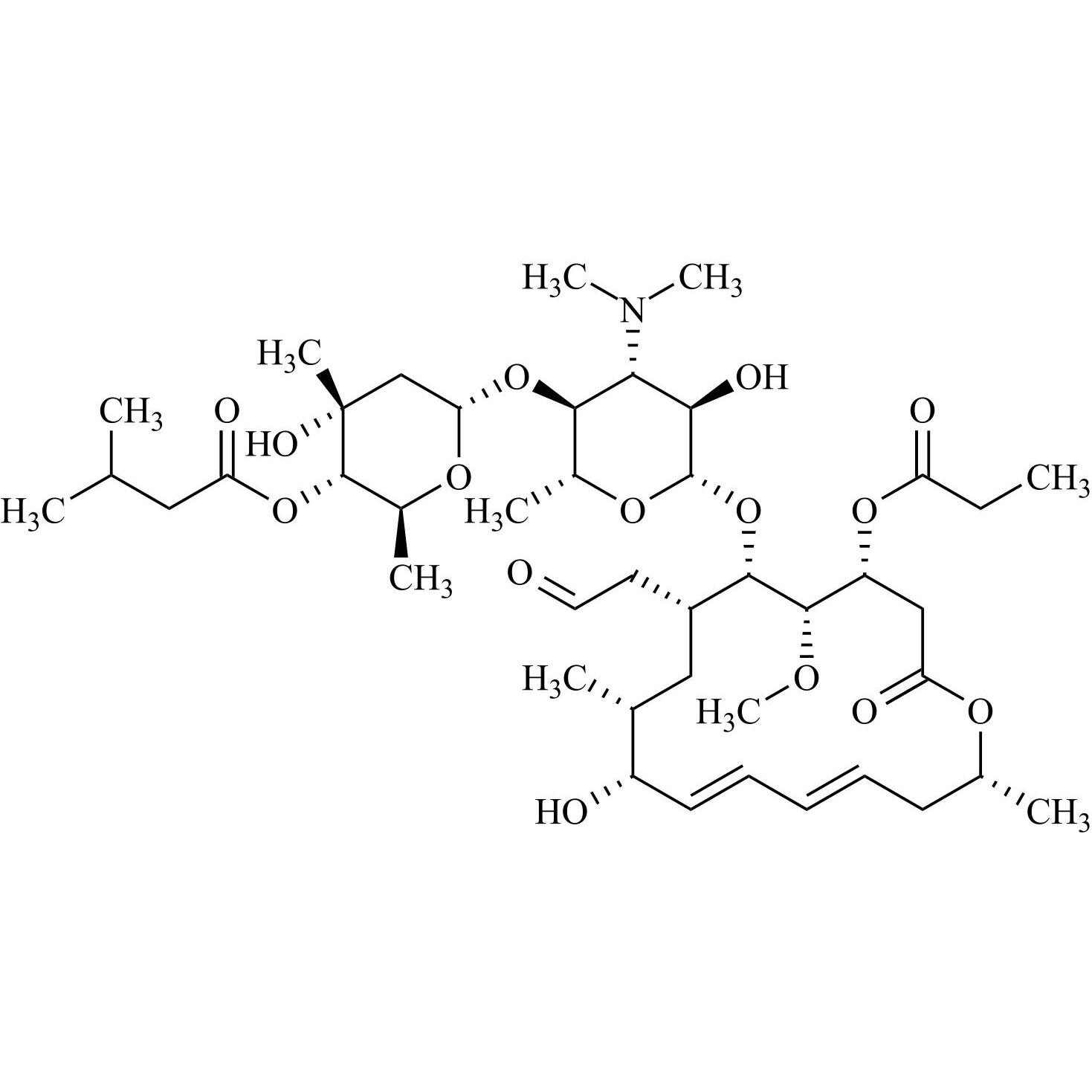
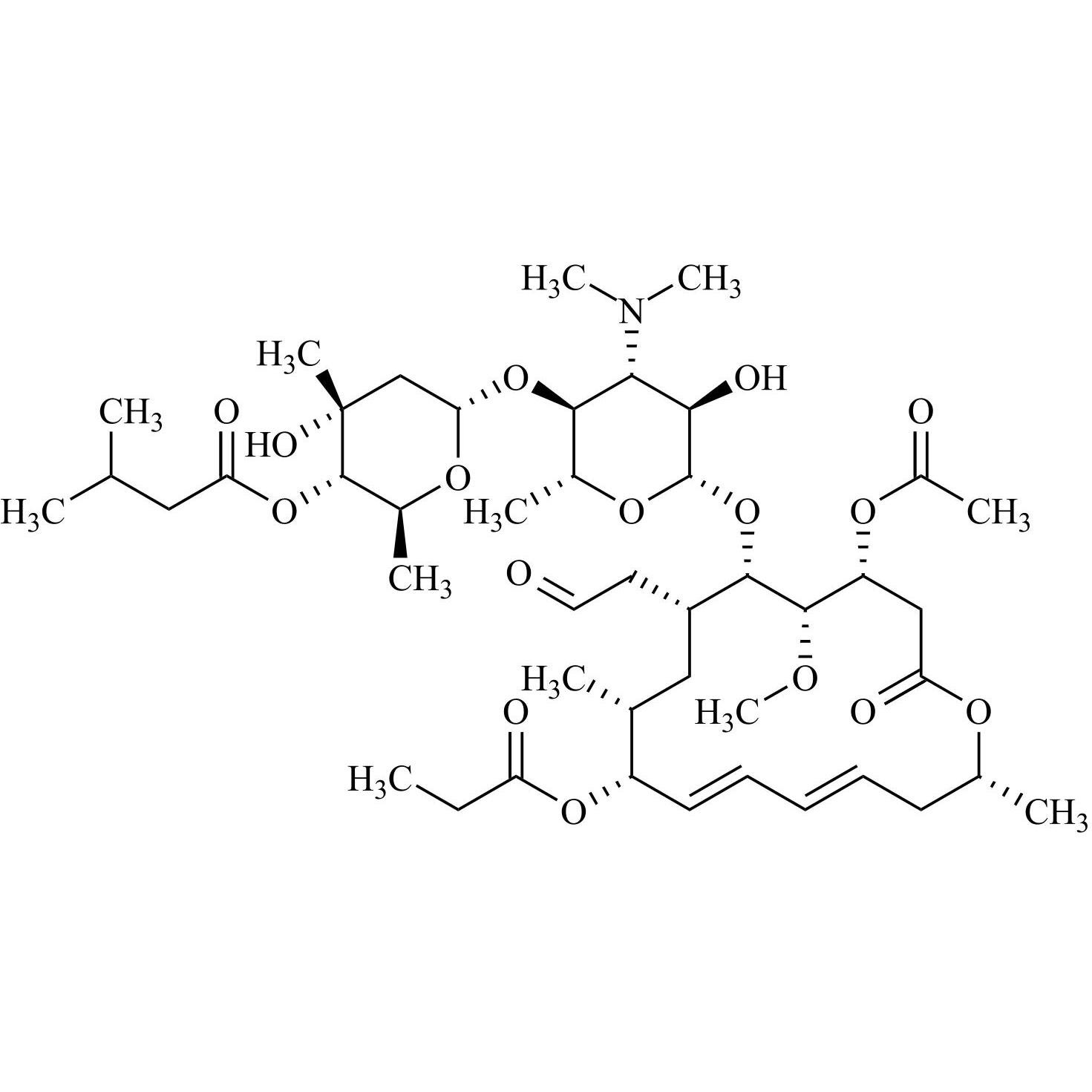
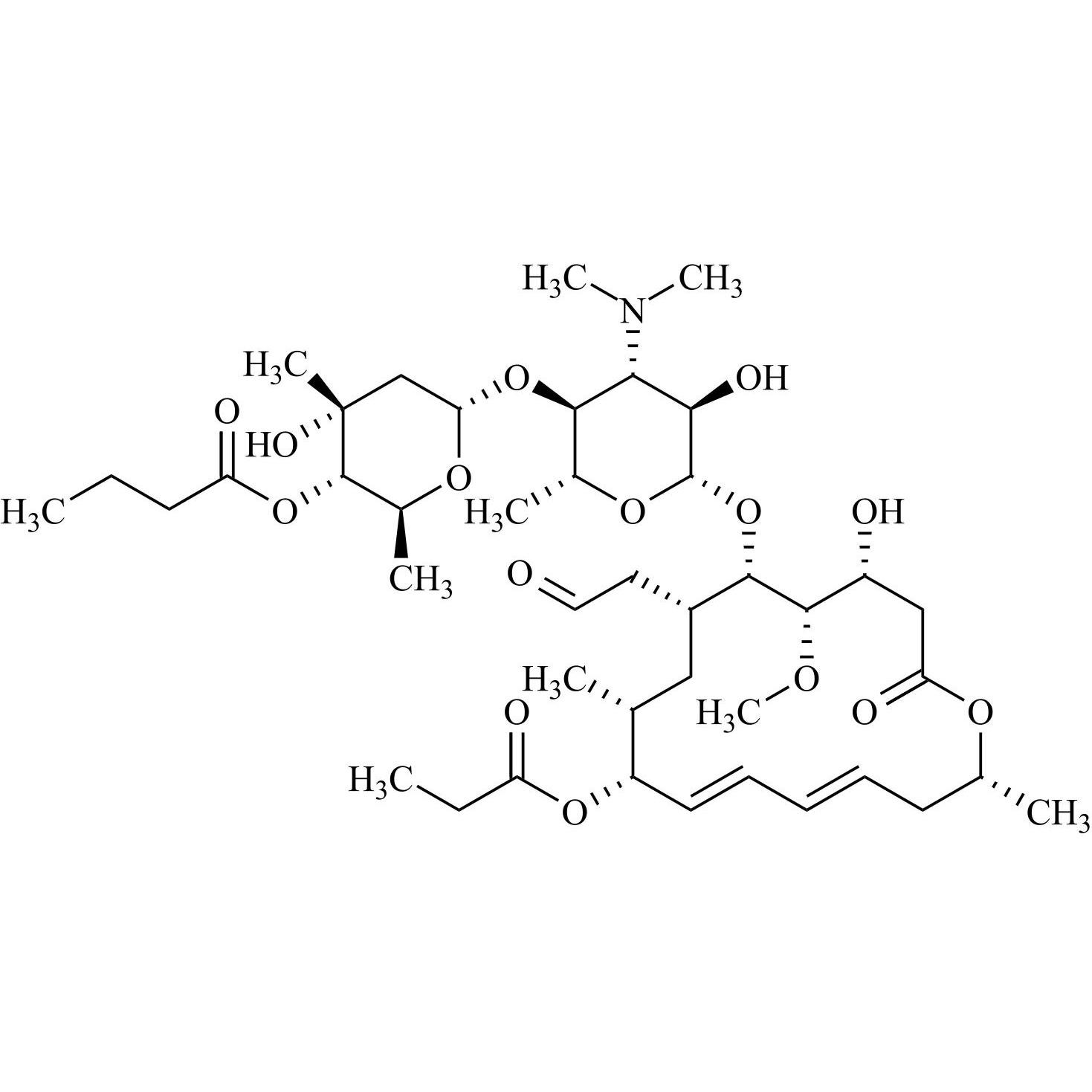
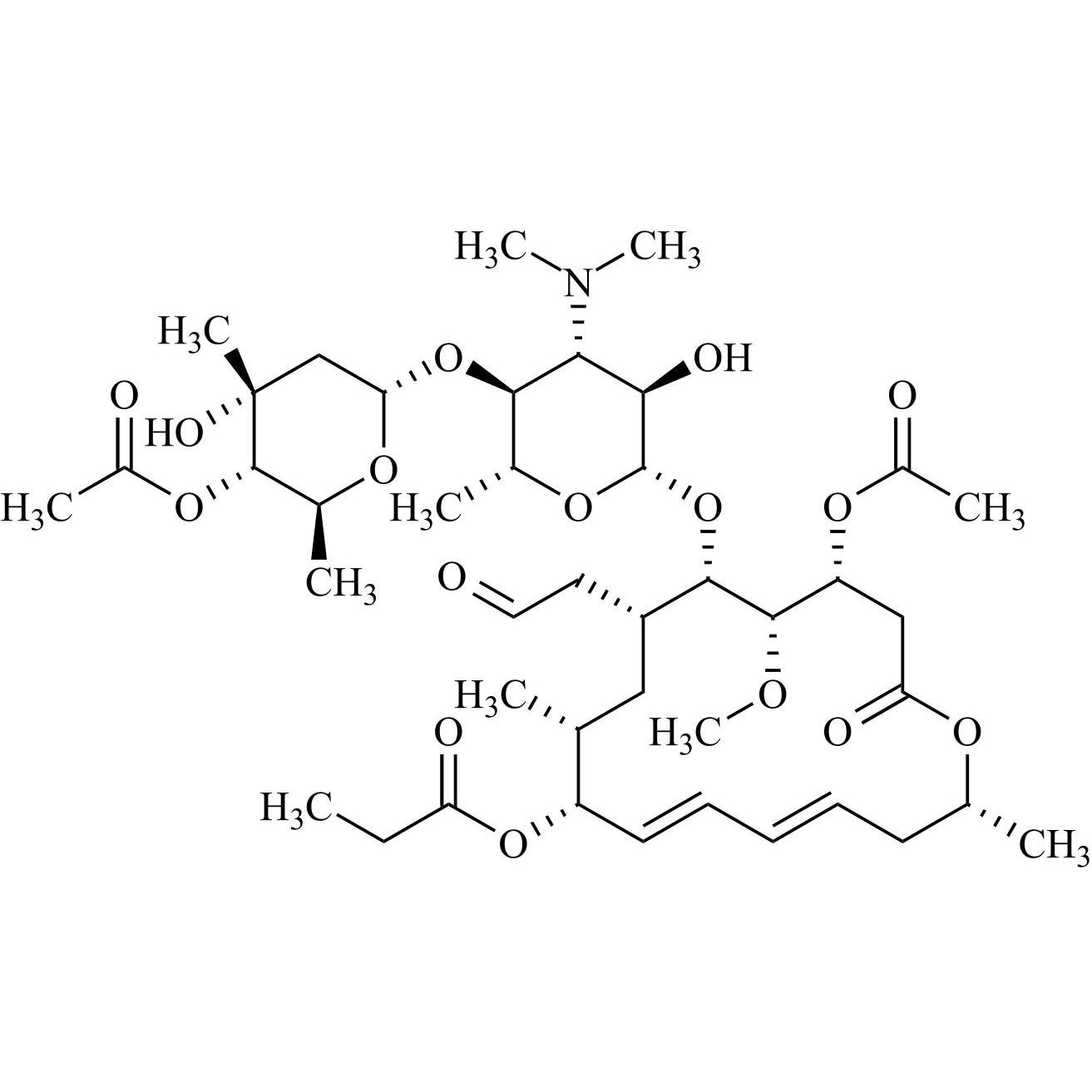
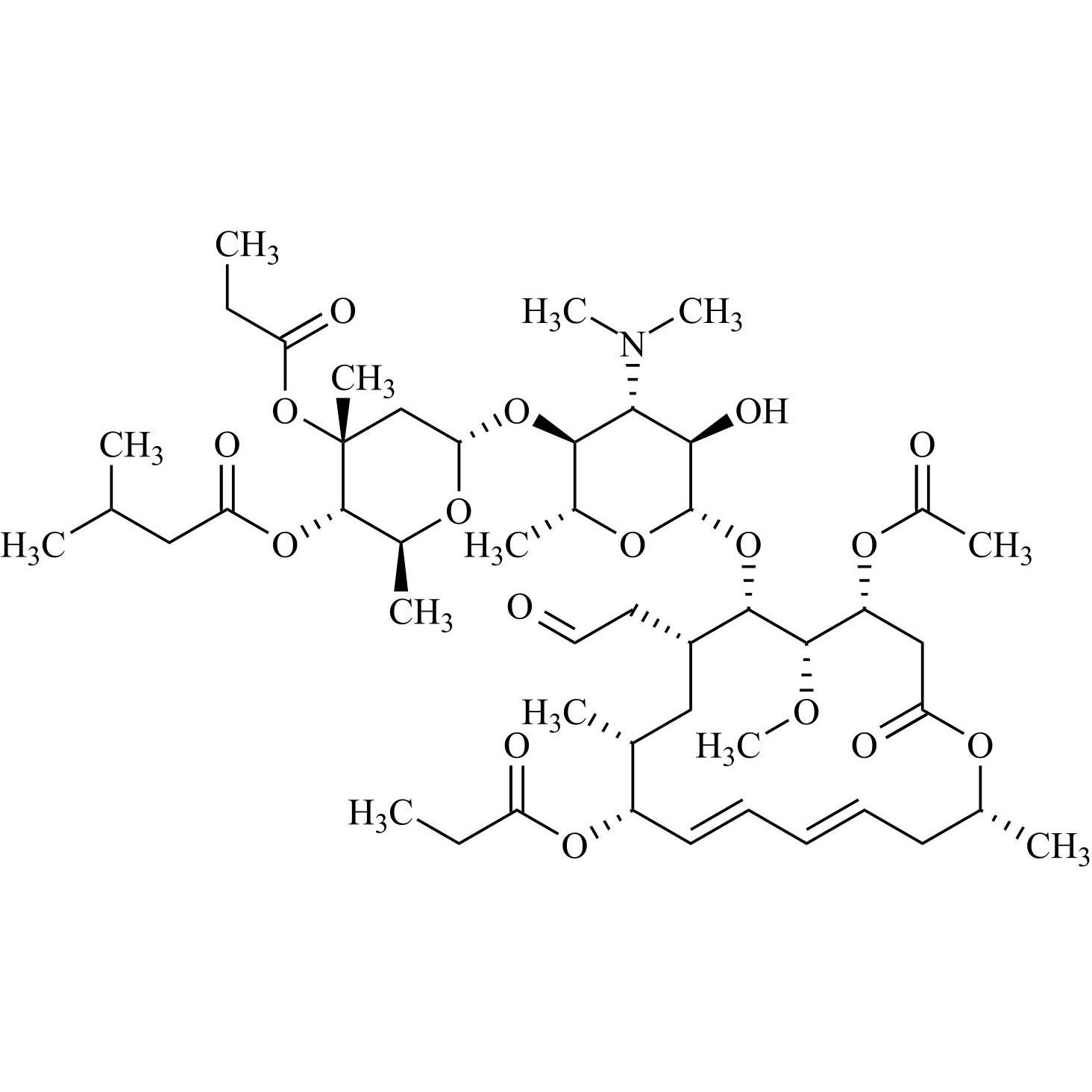
- Synonyms(4R,5S,6S,7R,9R,10R,11E,13E,16R)-4-(Acetyloxy)-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-4-O-butanoyl-3-C-methyl-α-L-ribo-hexopyranosyl)-3-(dimethylamino)-β-D-glucopyranosyl]oxy]-10-hydroxy-5-methoxy-9,16-dimethyl-7-(2-oxoethyl)oxacyclohexadeca-11,13-dien-2-one (as per EP)
- Description
(4R,5S,6S,7R,9R,10R,11E,13E,16R)-4-(Acetyloxy)-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-4-O-butanoyl-3-C-methyl-α-L-ribo-hexopyranosyl)-3-(dimethylamino)-β-D-glucopyranosyl]oxy]-10-hydroxy-5-methoxy-9,16-dimethyl-7-(2-oxoethyl)oxacyclohexadeca-11,13-dien-2-one (as per EP)
Josamycin EP Impurity A (Leucomycin A4) is a fully characterized chemical compound used as a reference standard of API Josamycin. The standard offered is compliant with regulatory guidelines. Josamycin EP Impurity A (Leucomycin A4) is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 18361-46-1