- Synonymsdibenzo(a,d)cycloheptadien-5-one;Dibenzo[a,d]cyclohepta[1,4]dien-5-one;Dibenzo[a,d]cycloheptadien-5-one;dibenzocycloheptenone;Dibenzosuberan-5-one;dibenzsuberone;dienone;dibenzo(b,f)cycloheptan-1-one;10,11-Dihydrodibenzo[a,d]cycloheptanone;10,11-Dihydrodibenzo[a,d]cyclohepten-5-one;2,3:6,7-Dibenz...
- Description
dibenzo(a,d)cycloheptadien-5-one;Dibenzo[a,d]cyclohepta[1,4]dien-5-one;Dibenzo[a,d]cycloheptadien-5-one;dibenzocycloheptenone;Dibenzosuberan-5-one;dibenzsuberone;dienone;dibenzo(b,f)cycloheptan-1-one;10,11-Dihydrodibenzo[a,d]cycloheptanone;10,11-Dihydrodibenzo[a,d]cyclohepten-5-one;2,3:6,7-Dibenzosuberone
Amitriptyline EP Impurity A (Dibenzosuberone) is a fully characterized chemical compound used as a reference standard of API Amitriptyline. The standard offered is compliant with regulatory guidelines. Amitriptyline EP Impurity A (Dibenzosuberone) is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 1210-35-1
Related products
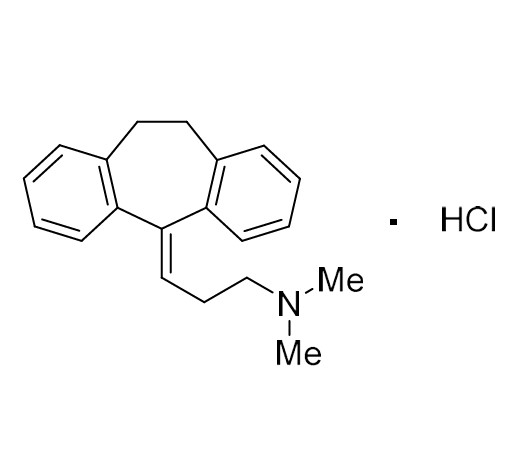
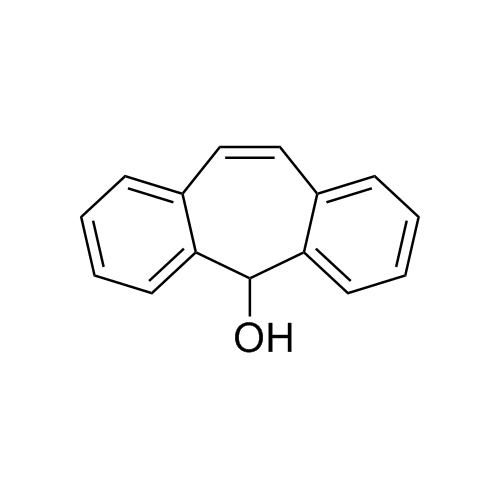
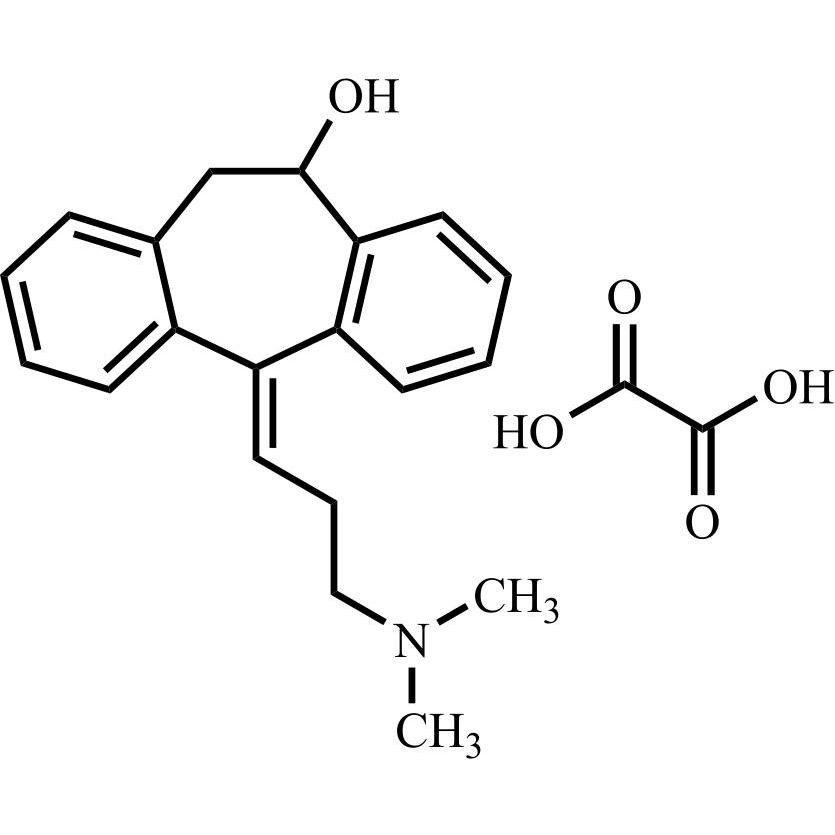
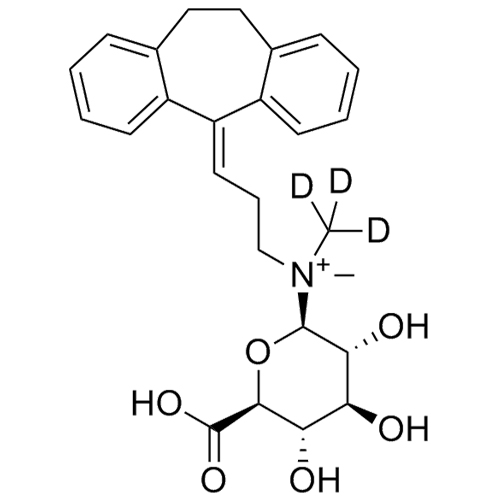
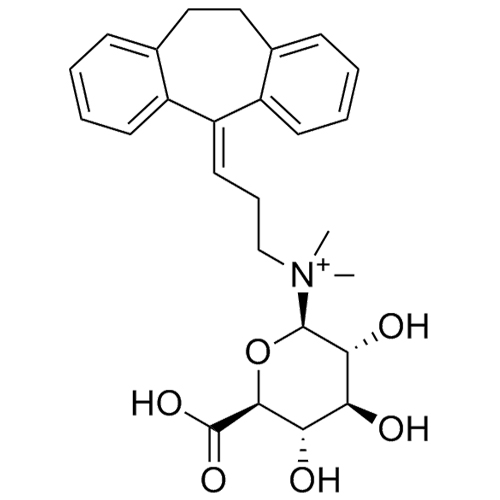
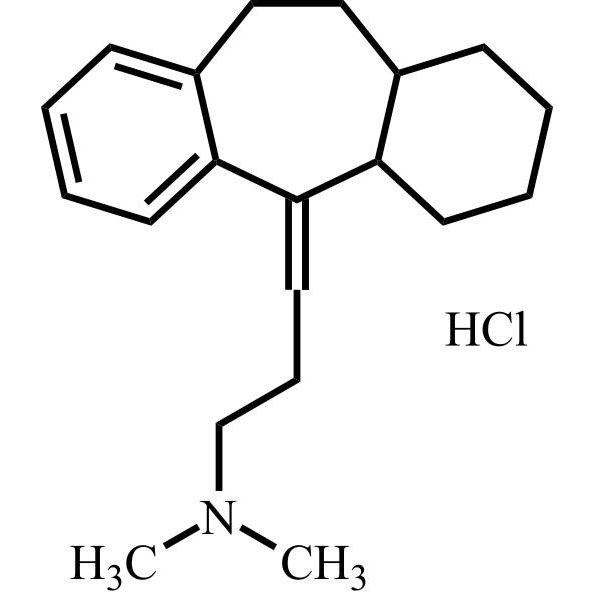
Amitriptyline EP Impurity E HCl (Mixture of Diastereomers)
M.F.
M.W. 283.46 36.46
CAT# AR-A01978
CAS# NA
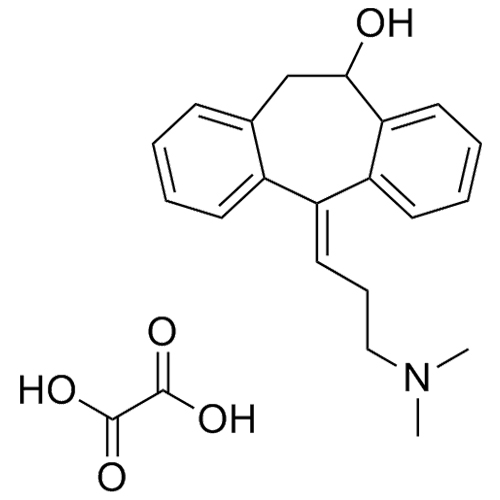
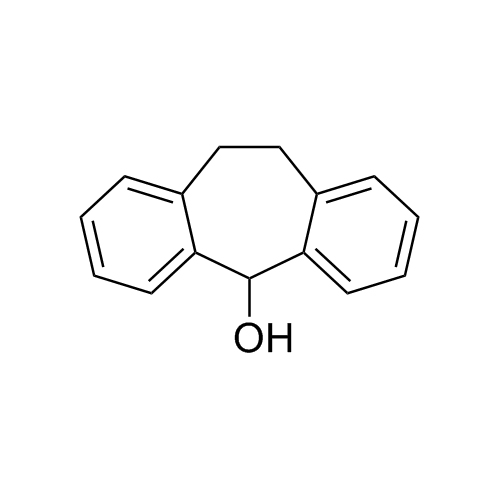
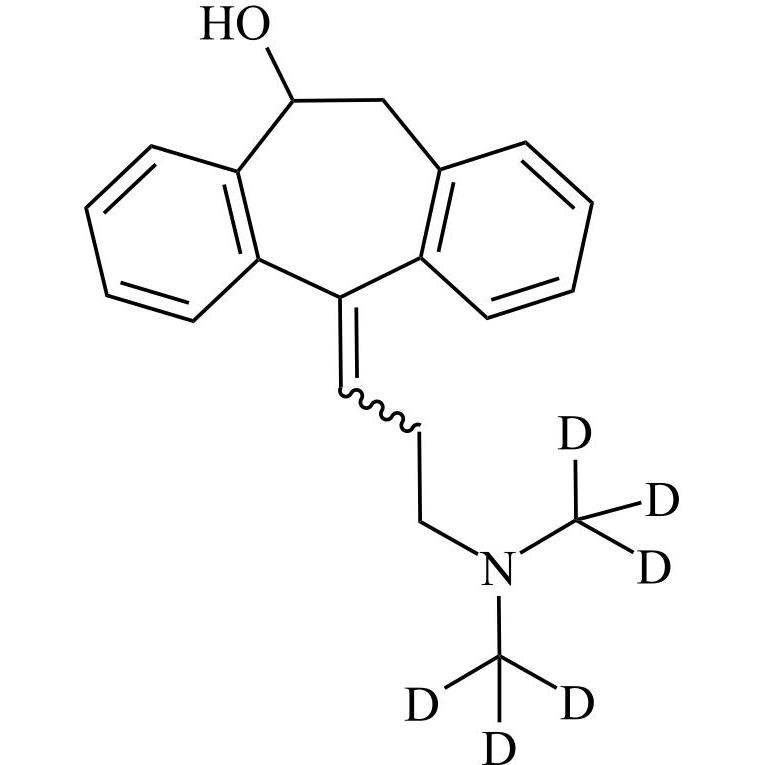
Amitriptyline EP Impurity F (Amitriptyline Hydroxy Impurity)
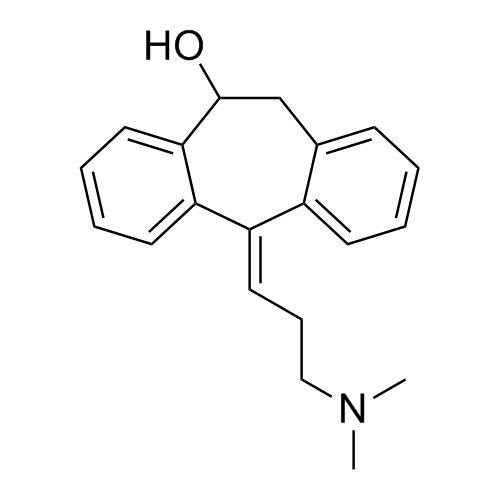
M.F.
M.W. 293.41
CAT# AR-A01977
CAS# 1159-82-6
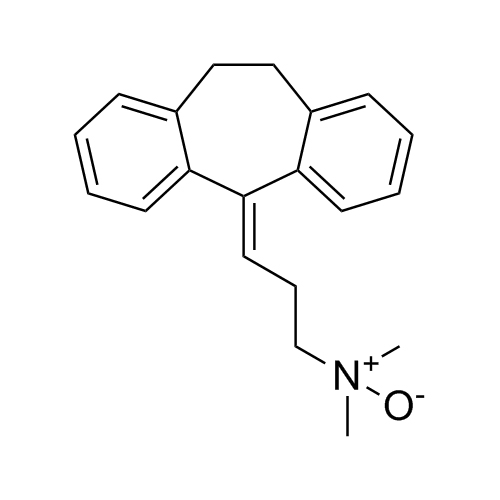
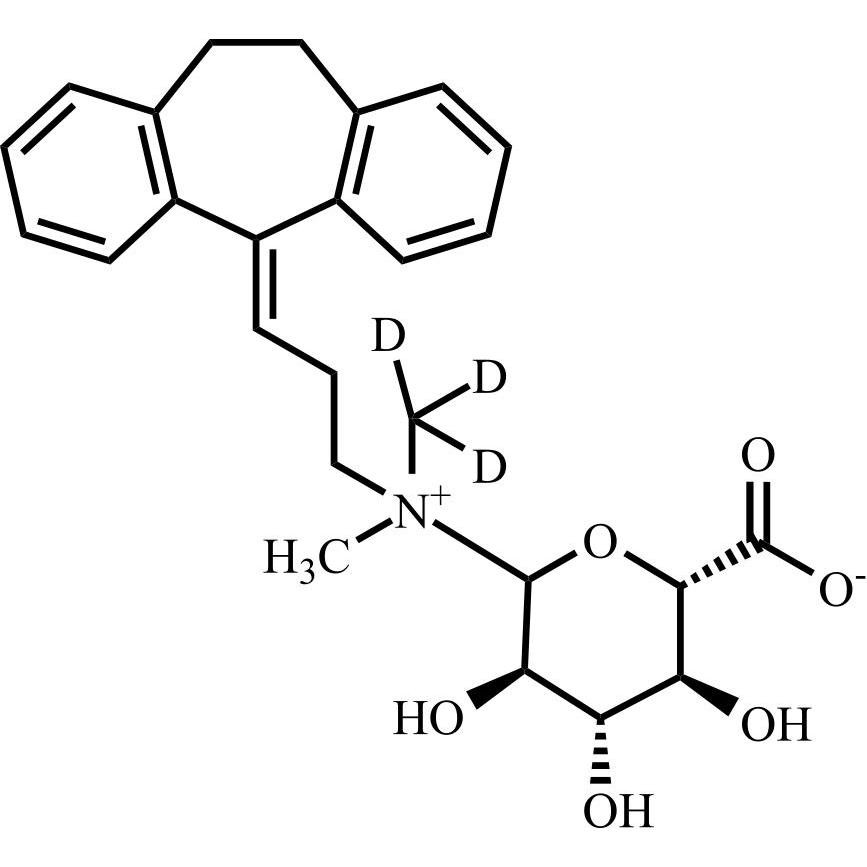
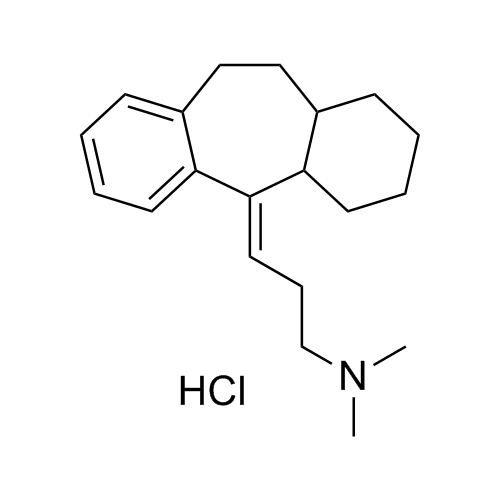
Amitriptyline EP Impurity E HCl (Mixture of Diastereomers)
M.F.
M.W. 283.46 36.46
CAT# AR-A06310
CAS# NA
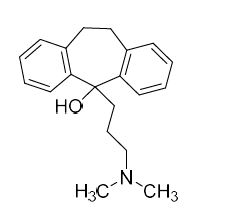
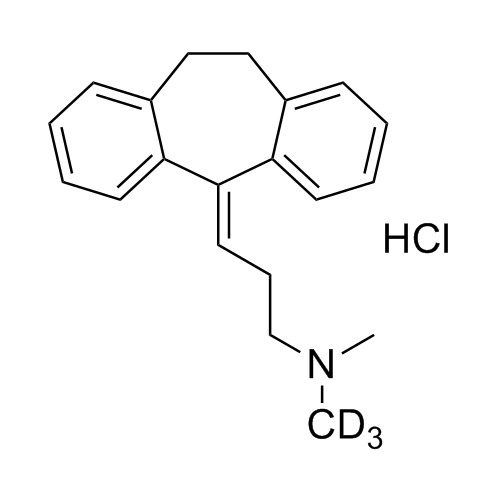
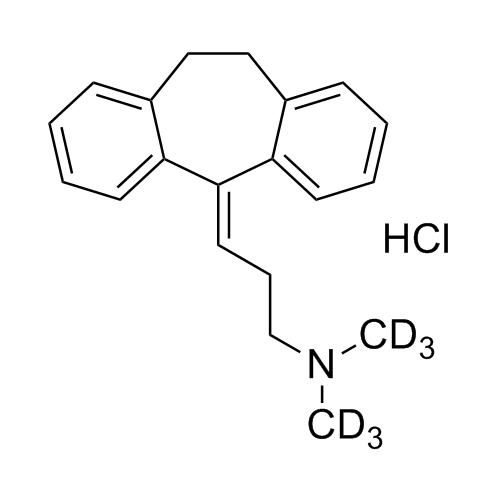
Amitriptyline-D3 HCl (Nortriptyline EP Impurity F-D3 HCl)

M.F.
M.W. 280.43 36.46
CAT# AR-A06311
CAS# 342611-00-1