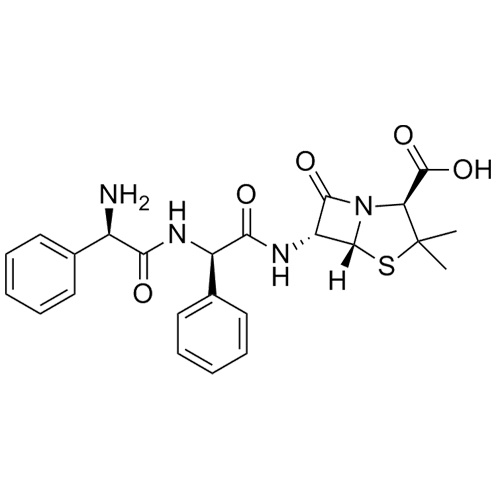
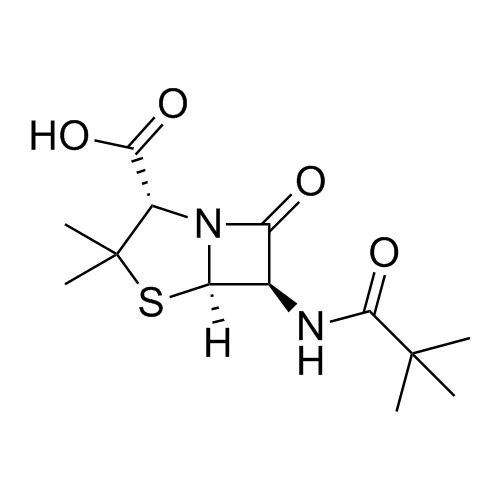
- Synonyms[2R-[2?(R*),4?]]-2-[[(Aminophenylacetyl)amino]methyl]-5,5-dimethyl-4-thiazolidinecarboxylic Acid; Ampicillin EP Impurity F; (2R,4S)-2-[[[(2R)-Aminophenylacetyl]amino]methyl]-5,5-dimethyl-4-thiazolidinecarboxylic Acid; (2R,4S)-2-[[[(2R)-2-Amino-2-phenylacetyl]amino]methyl]-5,5-dimethyl-4-thiazolid...
- Description
[2R-[2?(R*),4?]]-2-[[(Aminophenylacetyl)amino]methyl]-5,5-dimethyl-4-thiazolidinecarboxylic Acid; Ampicillin EP Impurity F; (2R,4S)-2-[[[(2R)-Aminophenylacetyl]amino]methyl]-5,5-dimethyl-4-thiazolidinecarboxylic Acid; (2R,4S)-2-[[[(2R)-2-Amino-2-phenylacetyl]amino]methyl]-5,5-dimethyl-4-thiazolidinecarboxylic Acid.
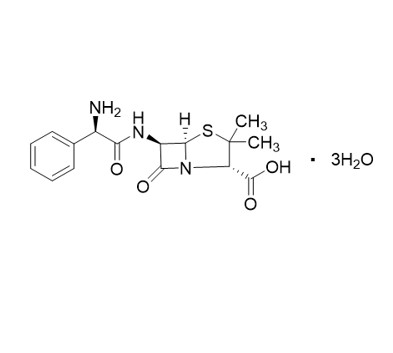
Ampicillin EP Impurity F is a fully characterized chemical compound used as a reference standard of API Ampicillin. The standard offered is compliant with regulatory guidelines. Ampicillin EP Impurity F is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 124774-48-7
Related products
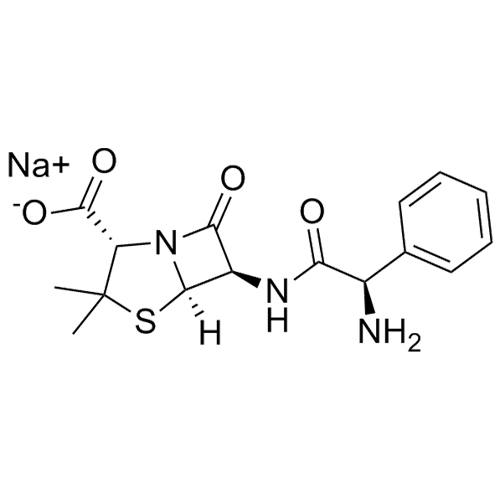
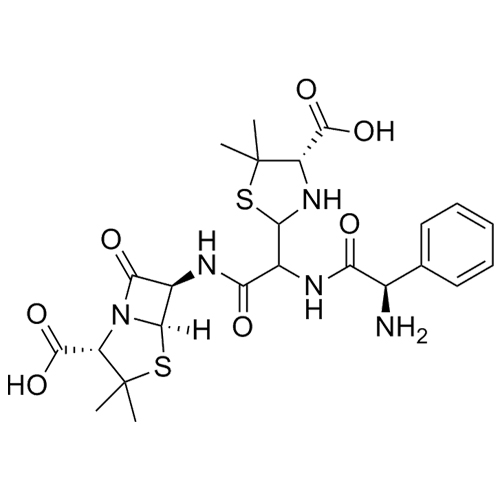
Ampicillin EP Impurity F Sodium Salt (Mixture of Diastereomers)
M.F.
M.W. 322.41 22.99
CAT# AR-A02116
CAS# NA
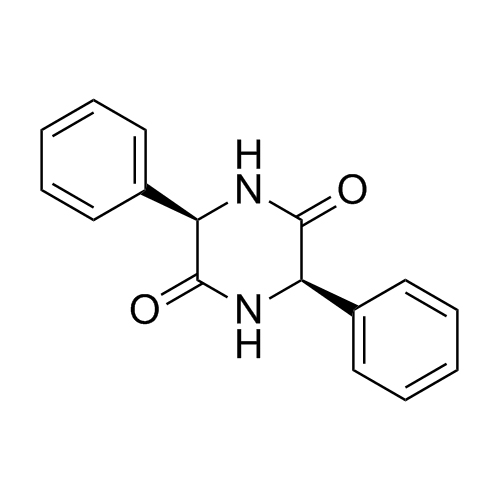
Ampicillin EP Impurity C (Ampicillin Diketopiperazine)
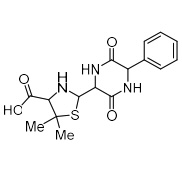
M.F.
M.W. 349.40
CAT# AR-A02113
CAS# 49841-96-5
Ampicillin EP Impurity D Disodium Salt (Mixture of Diastereomers)

M.F.
M.W. 365.41 2 22.99
CAT# AR-A02114
CAS# NA
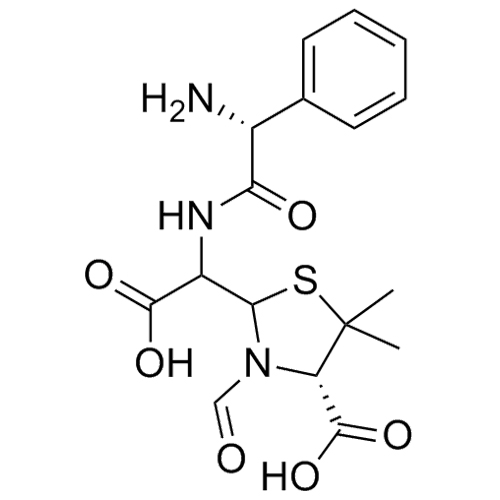
Ampicillin EP Impurity F (Mixture of Diastereomers)
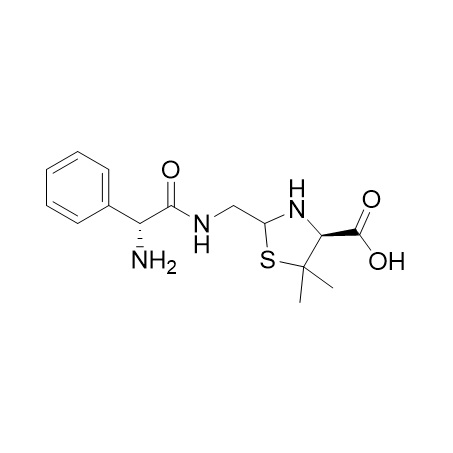
M.F.
M.W. 323.41
CAT# AR-A08064
CAS# 2197189-82-3 (relative stereochemistry)
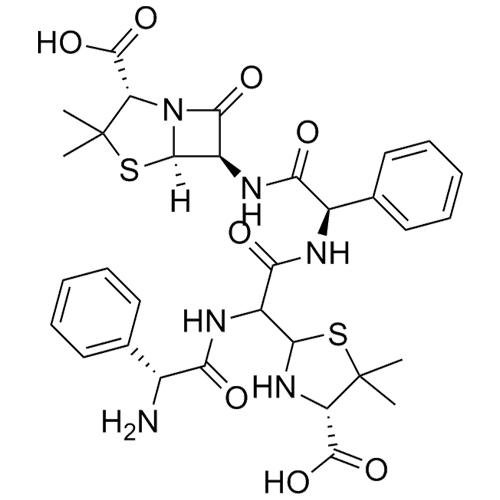
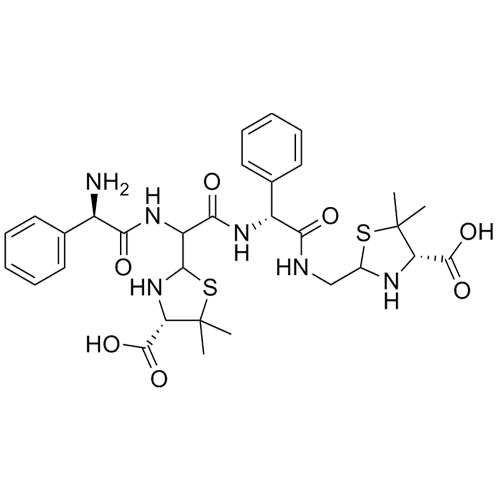
Ampicillin EP Impurity M Disodium Salt (Ampicillin Dimer Disodium Salt)

M.F.
M.W. 696.81 2*22.99
CAT# AR-A06485
CAS# NA
Ampicillin EP Impurity M Trisodium Salt (Ampicillin Trimer Trisodium Salt)

M.F.
M.W. 1045.19 3*22.99
CAT# AR-A06486
CAS# NA
Ampicillin EP Impurity D Disodium Salt (Mixture of Diastereomers)
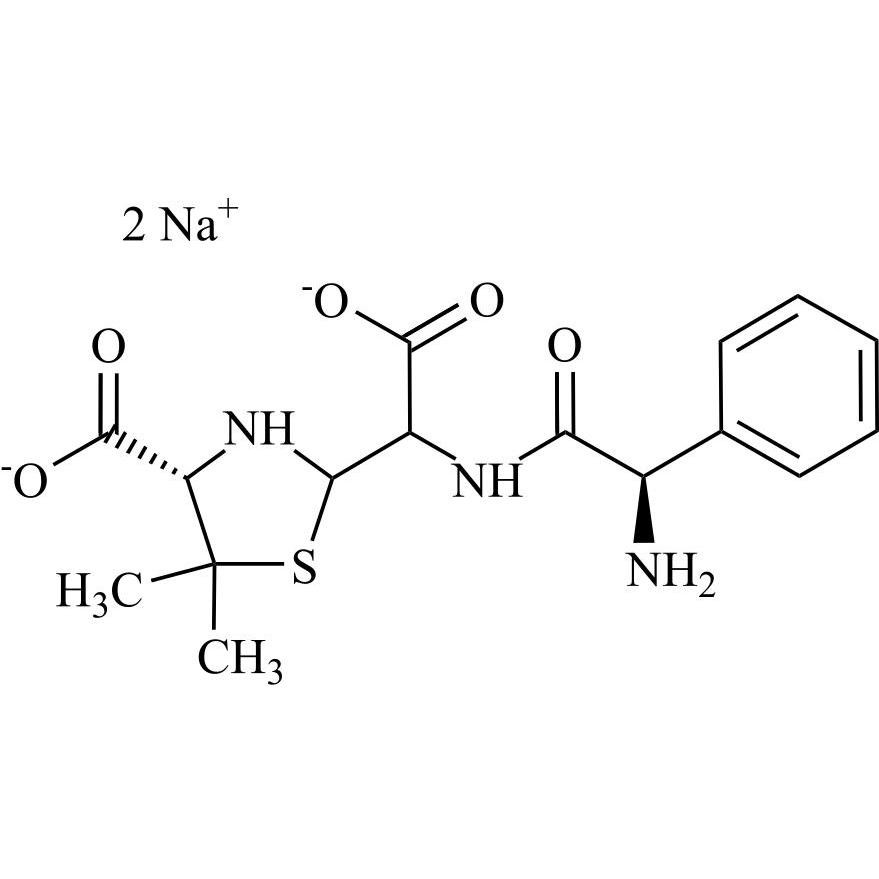
M.F.
M.W. 365.41 2*22.99
CAT# AR-A06494
CAS# NA