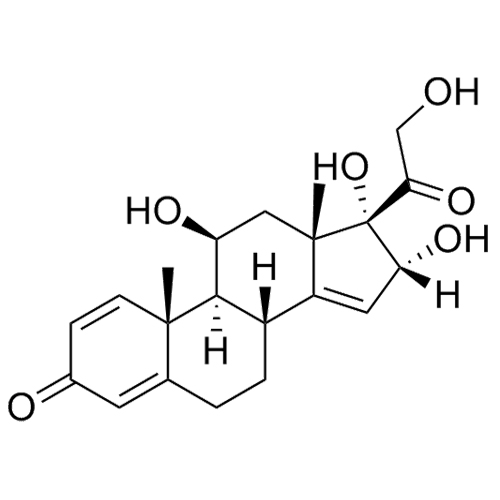
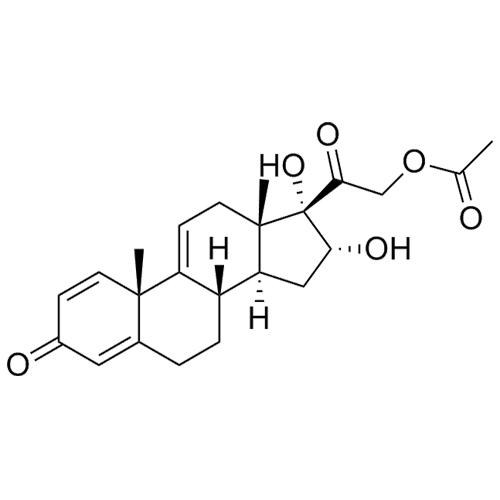
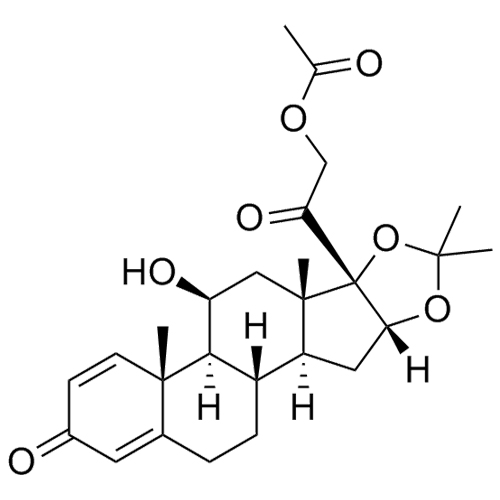
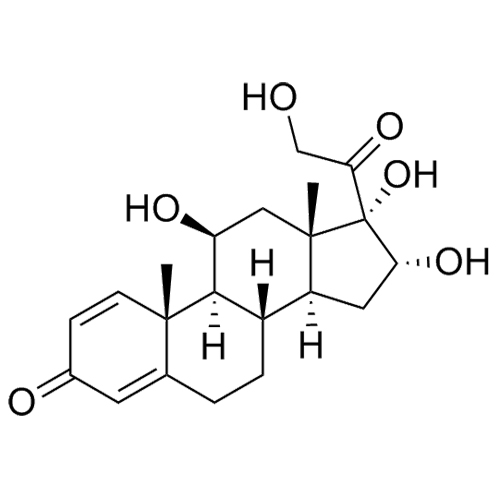
- Synonyms(8S,9S,10R,11S,13S,14S,16R,17S)-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one; 16?-Hydroxycortisol; U 34908; 16?,17-Dihydroxy-corticosterone;11?,16?,17,21-Tetrahydroxy-pregn-4-ene-3,20-dione; 11?,16?,17,21...
- Description
(8S,9S,10R,11S,13S,14S,16R,17S)-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one; 16?-Hydroxycortisol; U 34908; 16?,17-Dihydroxy-corticosterone;11?,16?,17,21-Tetrahydroxy-pregn-4-ene-3,20-dione; 11?,16?,17,21-Tetrahydroxypregn-4-ene-3,20-dione
Budesonide EP Impurity H is a fully characterized chemical compound used as a reference standard of API Budesonide. The standard offered is compliant with regulatory guidelines. Budesonide EP Impurity H is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 1171-81-9
Related products
6-alpha-Hydroxy Budesonide (Mixture of Diastereomers)

M.F.
M.W. 446.55
CAT# AR-B02158
CAS# 577777-51-6
6-beta-Hydroxy Budesonide (Mixture of Diastereomers)

M.F.
M.W. 446.54
CAT# AR-B02159
CAS# 88411-77-2
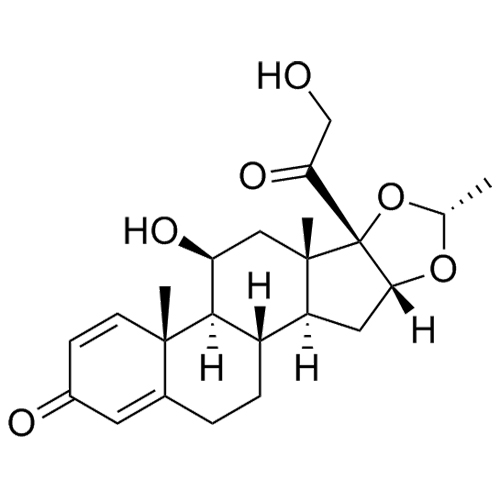
Budesonide EP Impurity H (Mixture of Diastereomers)

M.F.
M.W. 412.53
CAT# AR-B02178
CAS# 313474-58-7
Budesonide EP Impurity B (Mixture of Diastereomers)

M.F.
M.W. 402.49
CAT# AR-D01927
CAS# 1040085-98-0
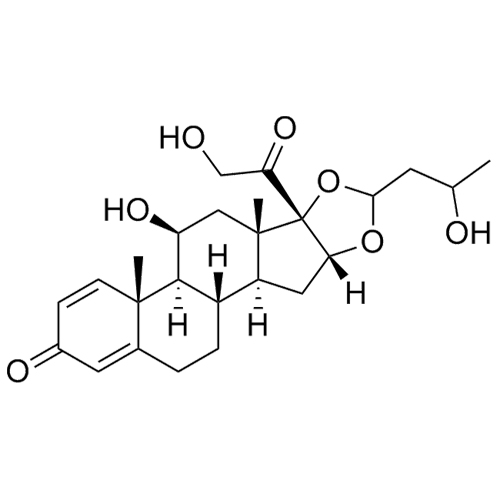
Budesonide EP Impurity J (Mixture of Diastereomers)

M.F.
M.W. 509.44
CAT# AR-B02176
CAS# 313474-59-8
Budesonide Sulfate Triethylamine Salt (Mixture of Diastereomers)

M.F.
M.W. 510.60 101.19
CAT# AR-B07948
CAS# 1436872-65-9