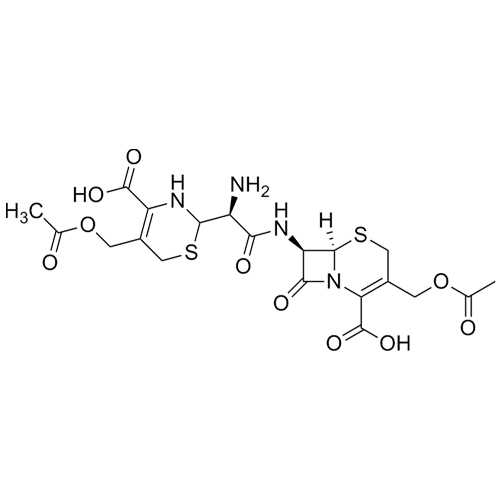
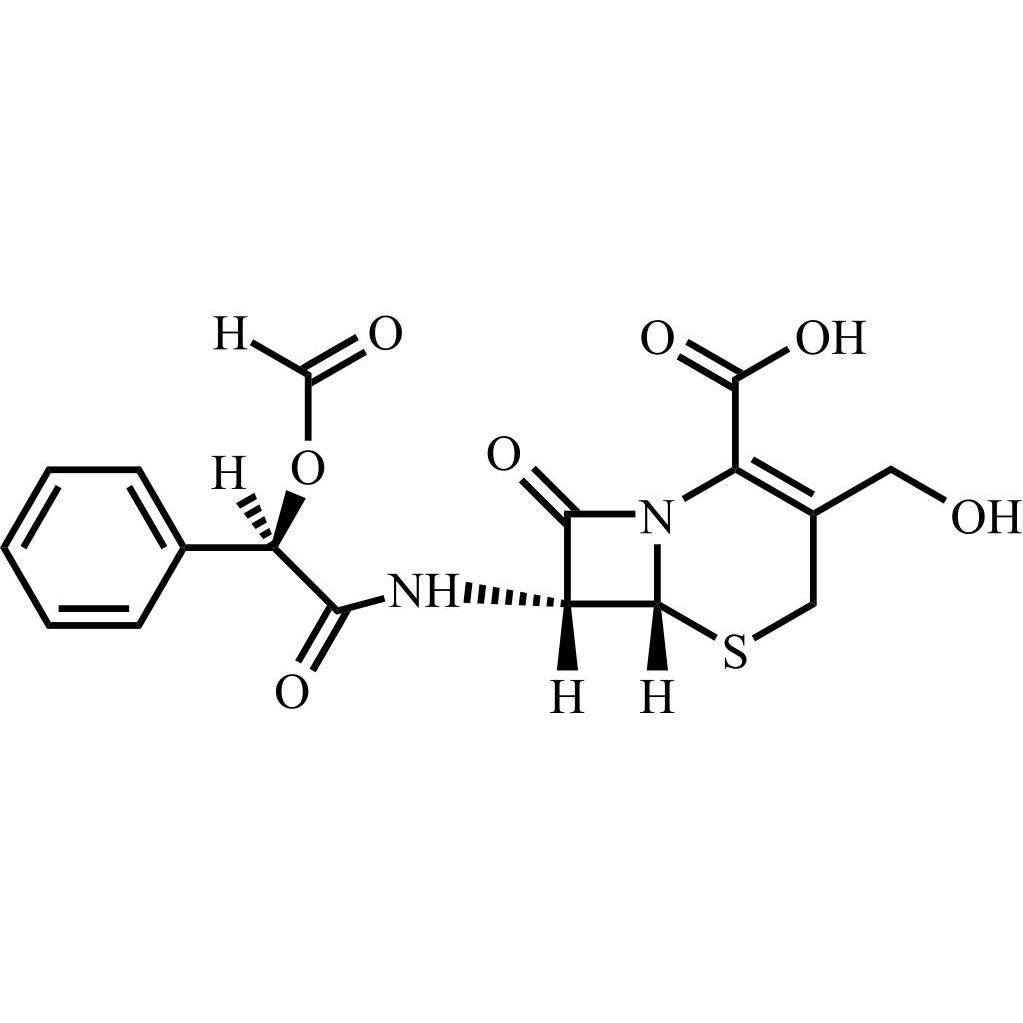
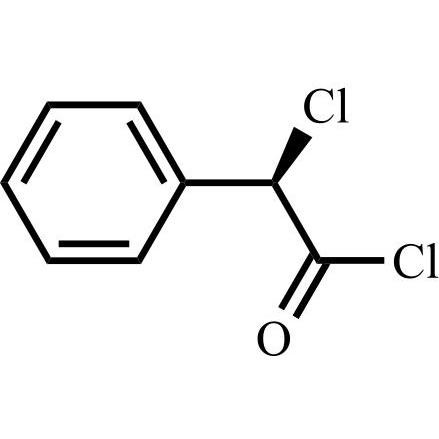
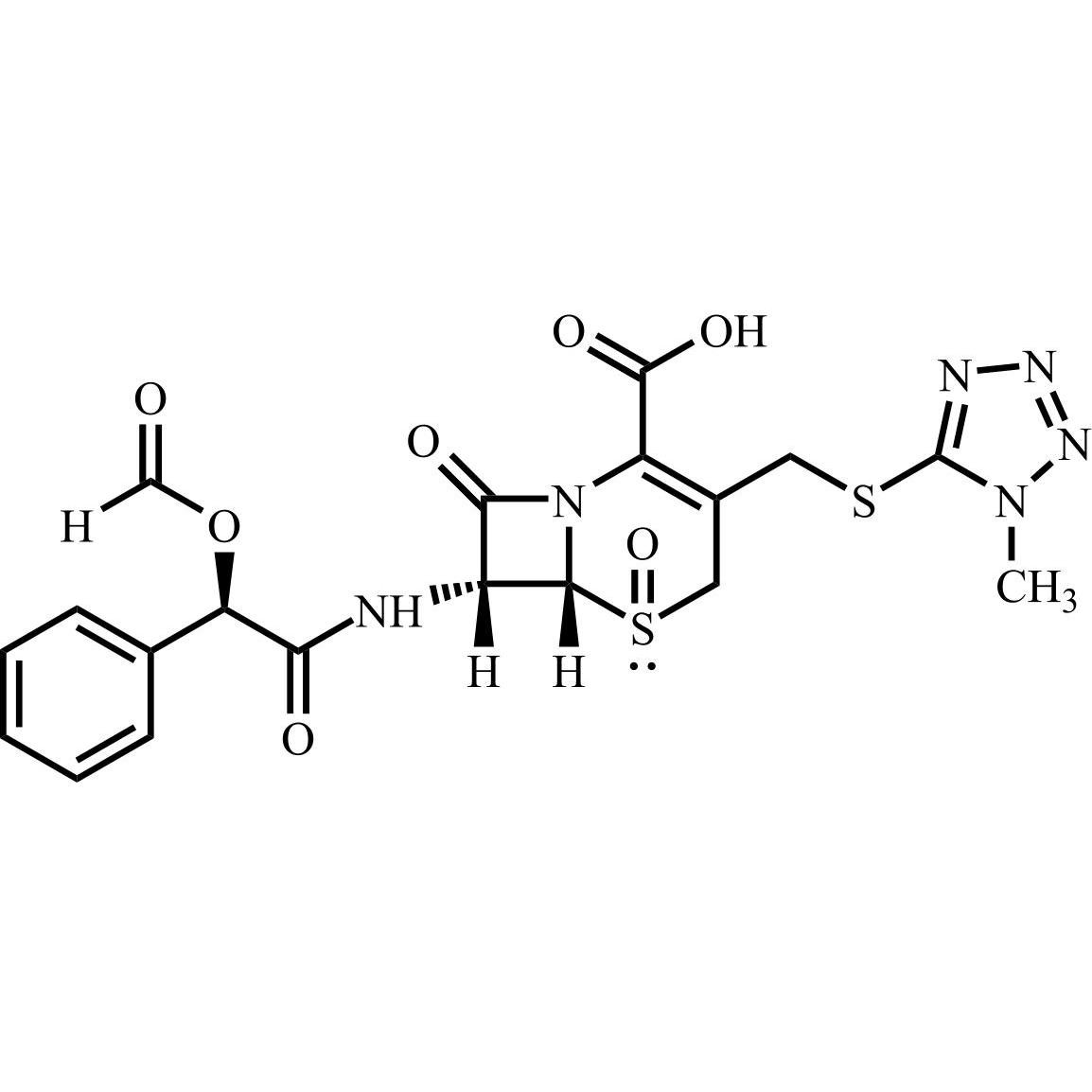
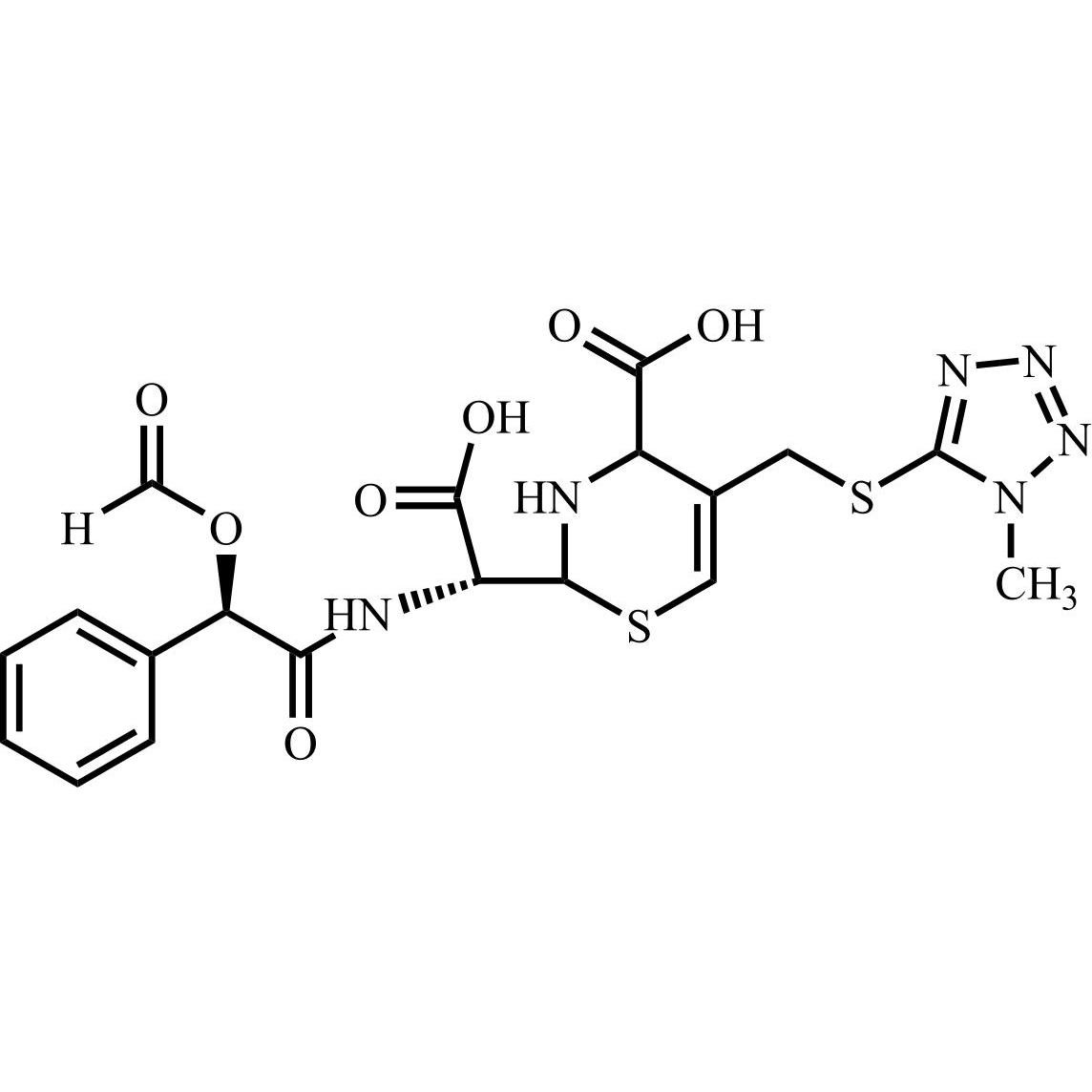
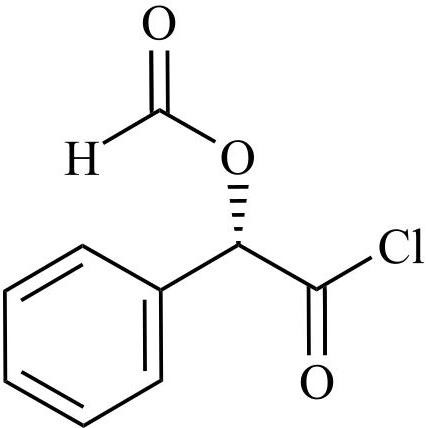
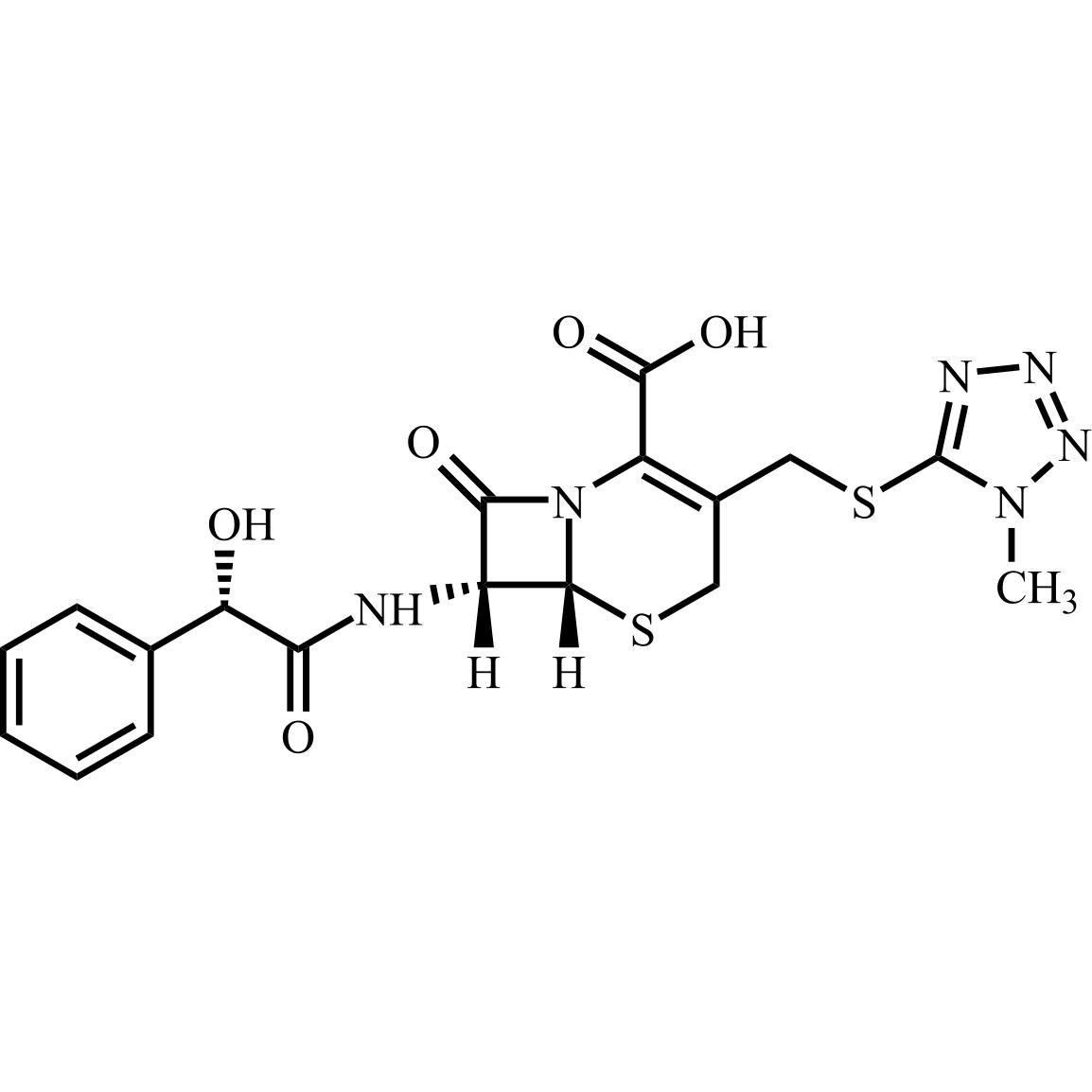
- Synonyms(6R,7R)-7-((R)-2-acetoxy-2-phenylacetamido)-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, sodium salt
- Description
(6R,7R)-7-((R)-2-acetoxy-2-phenylacetamido)-3-(((1-methyl-1H-tetrazol-5-yl)thio)methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, sodium salt
Cefamandole EP Impurity C Sodium Salt is a fully characterized chemical compound used as a reference standard of API Cefamandole. The standard offered is compliant with regulatory guidelines. Cefamandole EP Impurity C Sodium Salt is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 36922-16-4