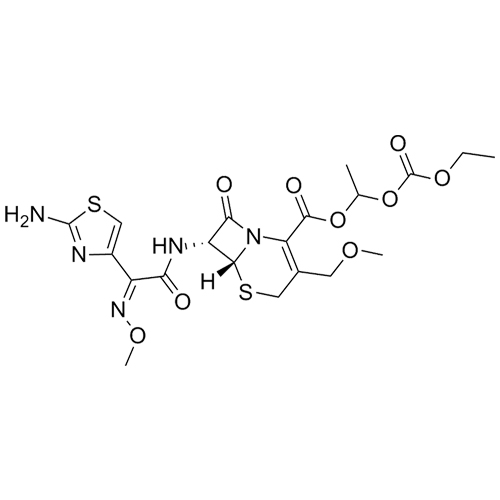
- Synonyms(6R,7R)-1-((isopropoxycarbonyl)oxy)ethyl 7-((E)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate 5-oxide
- Description
(6R,7R)-1-((isopropoxycarbonyl)oxy)ethyl 7-((E)-2-(2-aminothiazol-4-yl)-2-(methoxyimino)acetamido)-3-(methoxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate 5-oxide
Cefpodoxime Proxetil Impurity K (Mixture of Diastereomers) is a fully characterized chemical compound used as a reference standard of API Cefpodoxime Proxetil. The standard offered is compliant with regulatory guidelines. Cefpodoxime Proxetil Impurity K (Mixture of Diastereomers) is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS -
Related products
Cefpodoxime Proxetil EP Impurity B (Mixture of Diastereomers)

M.F.
M.W. 527.58
CAT# AR-C01984
CAS# 947692-14-0
Cefpodoxime Proxetil EP Impurity F (Mixture of Diastereomers)

M.F.
M.W. 585.6
CAT# AR-C01988
CAS# 96680-30-7
Cefpodoxime Proxetil EP Impurity G (Mixture of Diastereomers)

M.F.
M.W. 599.63
CAT# AR-C01989
CAS# 947692-15-1
Cefpodoxime Proxetil EP Impurity A (Cefpodoxime Acid)

M.F.
M.W. 427.45
CAT# AR-C01981
CAS# 80210-62-4
Cefpodoxime Proxetil EP Impurity A -d3 Sodium Salt (Cefpodoxime-d3 Acid Sodium Salt)

M.F.
M.W. 429.47 22.99
CAT# AR-C01982
CAS# 82619-04-3(non-labelled)
Cefpodoxime Proxetil EP Impurity A -d3 Sodium Salt (Cefpodoxime-d3 Acid Sodium Salt)
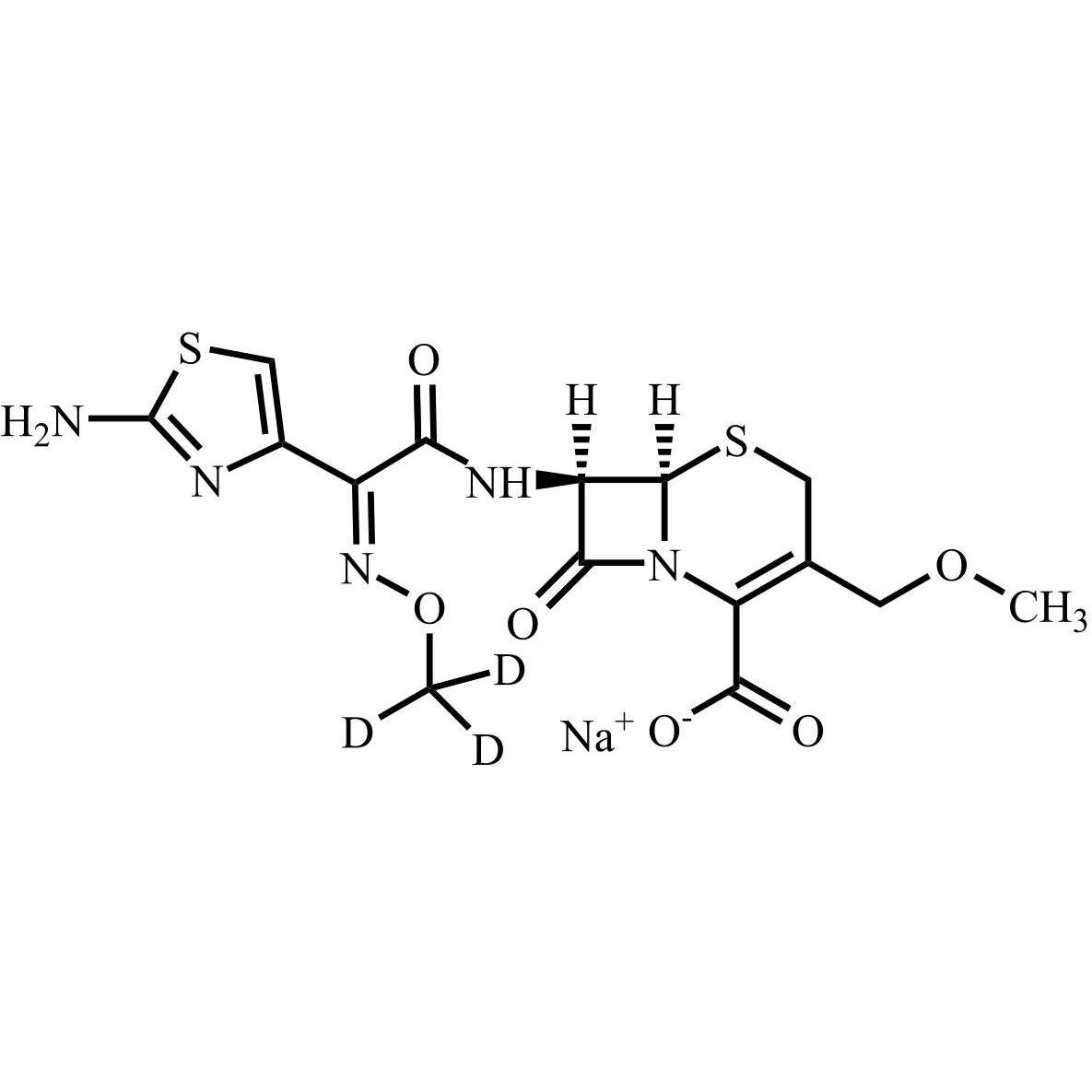
M.F.
M.W. 429.47 22.99
CAT# AR-C06741
CAS# NA