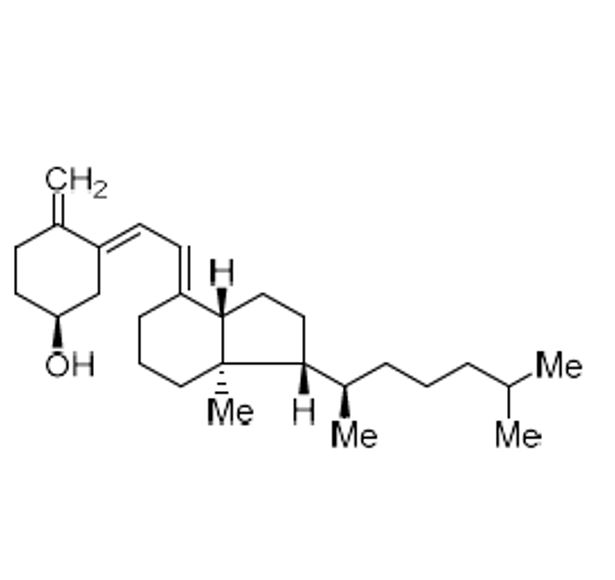
- Synonyms(S)-4-methyl-3-((E)-2-((1R,7aR)-7a-methyl-1-((R)-6-methylheptan-2-yl)-2,3,5,6,7,7a-hexahydro-1H-inden-4-yl)vinyl)cyclohex-3-enol; Isotachysterol3; (3?,6E)-9,10-Secocholesta-5(10),6,8(14)-trien-3-ol; (E)-9,10-Secocholesta-5(10),6,8(14)-trien-3?-ol; Toxisterol3 D3; Cholecalciferol EP Impurity D
- Description
(S)-4-methyl-3-((E)-2-((1R,7aR)-7a-methyl-1-((R)-6-methylheptan-2-yl)-2,3,5,6,7,7a-hexahydro-1H-inden-4-yl)vinyl)cyclohex-3-enol; Isotachysterol3; (3?,6E)-9,10-Secocholesta-5(10),6,8(14)-trien-3-ol; (E)-9,10-Secocholesta-5(10),6,8(14)-trien-3?-ol; Toxisterol3 D3; Cholecalciferol EP Impurity D
Cholecalciferol EP Impurity D (iso-Tachysterol 3) is a fully characterized chemical compound used as a reference standard of API Cholecalciferol. The standard offered is compliant with regulatory guidelines. Cholecalciferol EP Impurity D (iso-Tachysterol 3) is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 22350-43-2
Related products
Cholecalciferol EP Impurity F (Cholecalciferol butyrate)
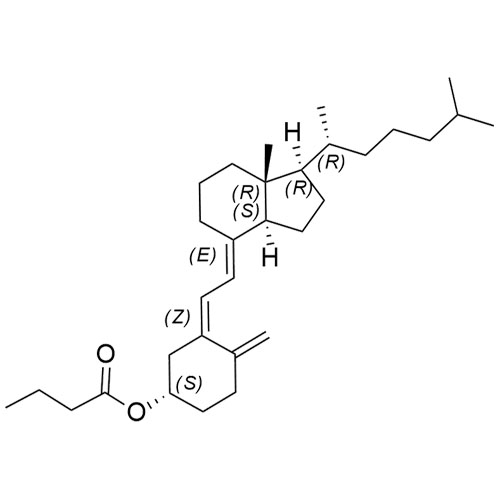
M.F.
M.W. 454.73
CAT# AR-C03990
CAS# 31316-20-8
Cholecalciferol EP Impurity A (5,6-trans-Cholecalciferol,5,6-trans-Vitamin D3)
M.F.
M.W. 384.65
CAT# AR-C02576
CAS# 22350-41-0
Cholecalciferol Sulfate Triethylamine Salt (Vitamin D3 Sulfate Triethylamine Salt)

M.F.
M.W. 464.71 101.19
CAT# AR-C07283
CAS# NA



















