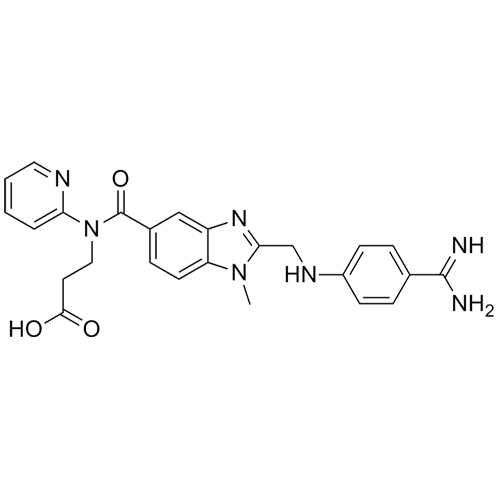
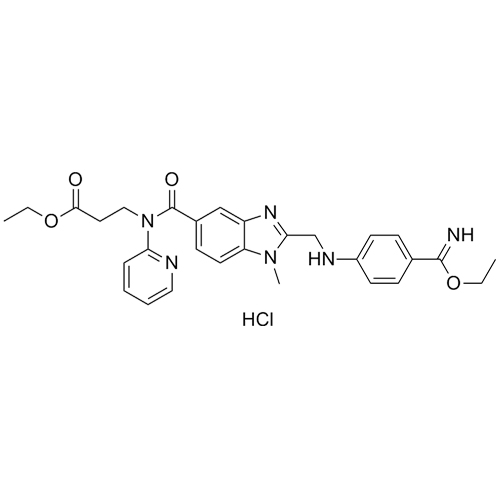
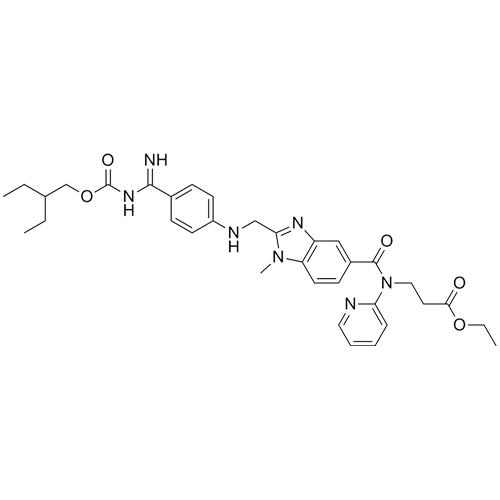
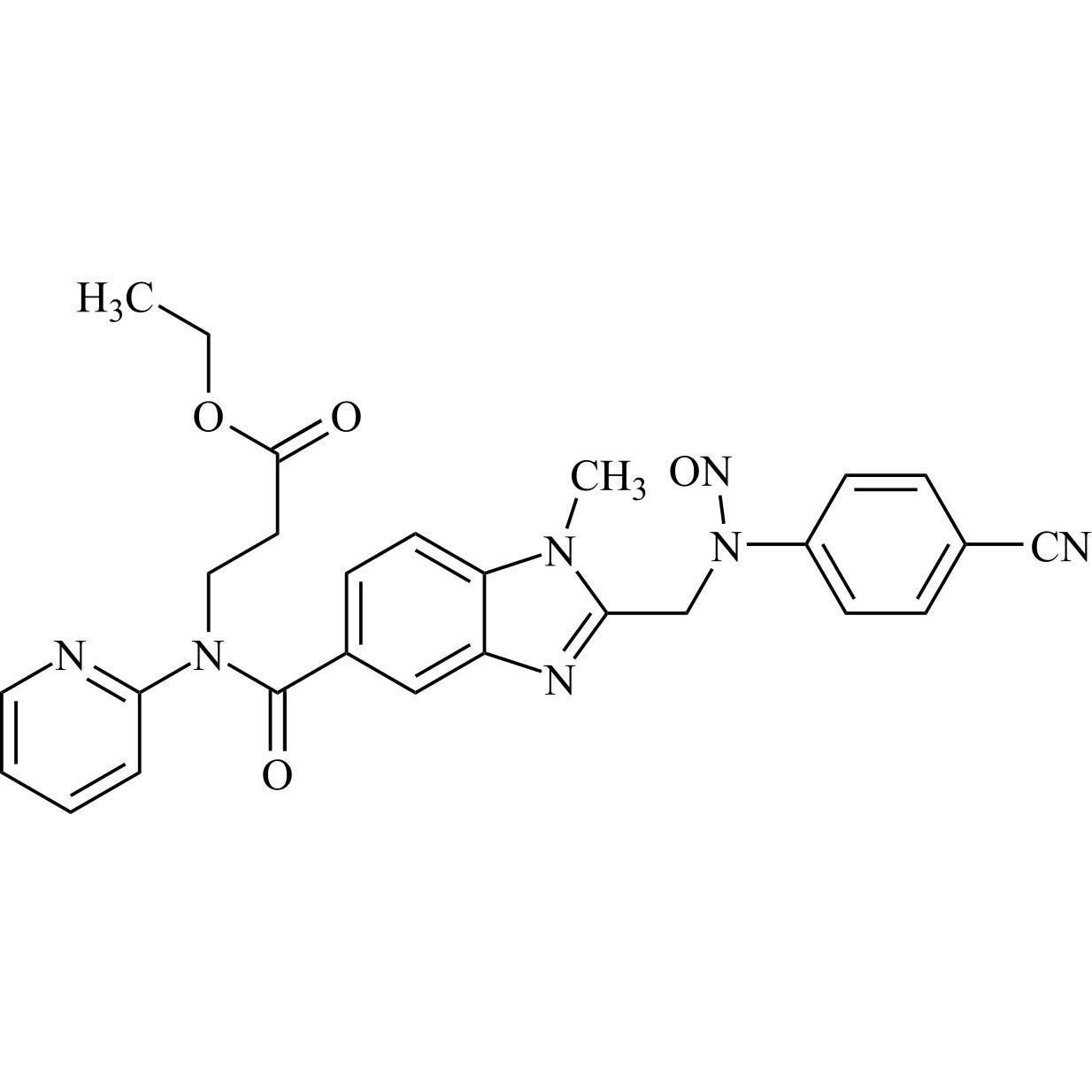
- Synonymsethyl 3-(2-(((4-cyanophenyl)amino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzo[d]imidazole-5-carboxamido)propanoate;N-[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-?-alanine Ethyl Ester;Deacetamidine Cyano Dabigatran Ethyl Ester
- Description
ethyl 3-(2-(((4-cyanophenyl)amino)methyl)-1-methyl-N-(pyridin-2-yl)-1H-benzo[d]imidazole-5-carboxamido)propanoate;N-[[2-[[(4-Cyanophenyl)amino]methyl]-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridinyl-?-alanine Ethyl Ester;Deacetamidine Cyano Dabigatran Ethyl Ester
Dabigatran impurity I is a fully characterized chemical compound used as a reference standard of API Dabigatran. The standard offered is compliant with regulatory guidelines. Dabigatran impurity I is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 211915-84-3
Related products
Dabigatran Etexilate Impurity 10 (2-Heptyl Chloroformate)
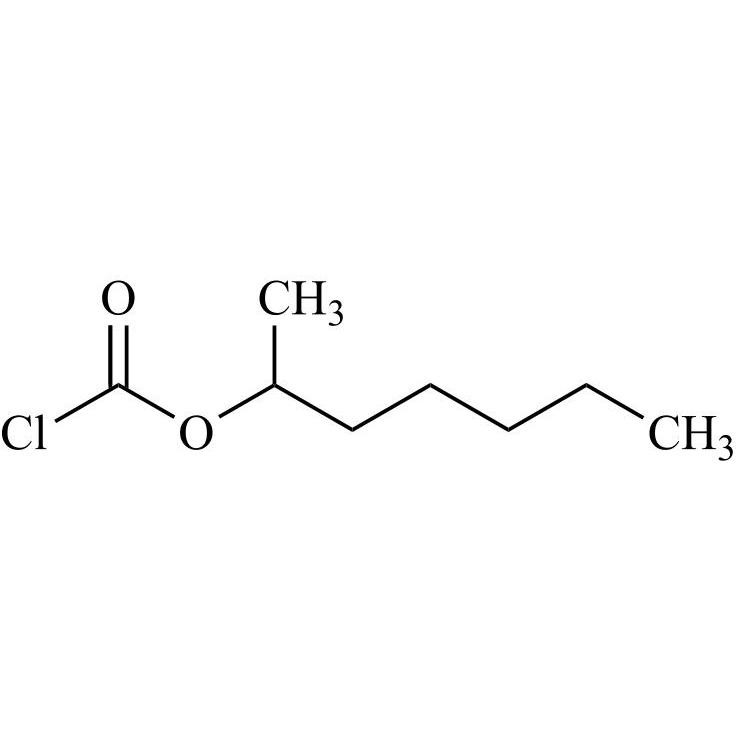
M.F.
M.W. 178.66
CAT# AR-D06166
CAS# 290819-03-3
Dabigatran Acyl-beta-D-Glucuronide-d3
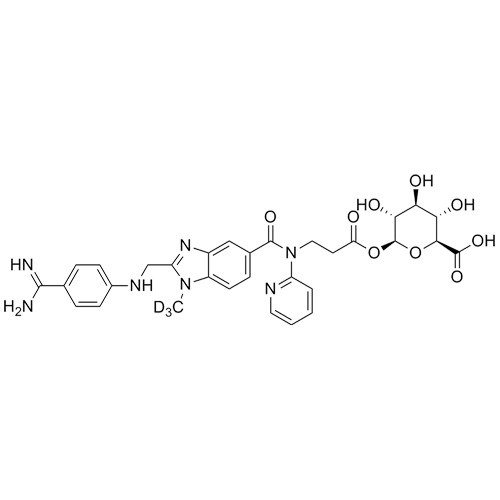
M.F.
M.W. 650.66
CAT# AR-D01060
CAS# 1015167-40-4 (non-labelled)
Dabigatran Acyl-O-2-D-Glucuronide Trifluoroacetic Acid Salt
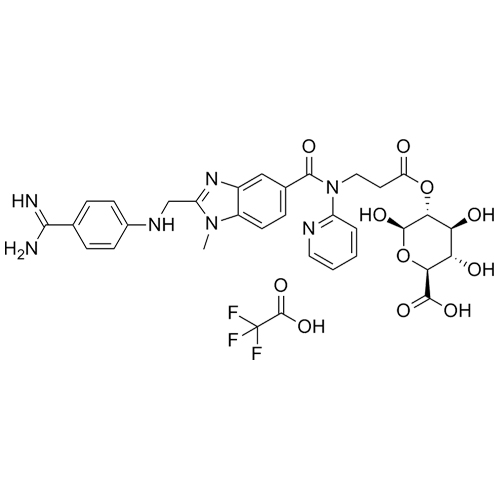
M.F.
M.W. 647.65 114.02
CAT# AR-D01055
CAS# 1296162-13-4 (free base)
Dabigatran Acyl-O-3-D-Glucuronide Trifluoroacetic Acid Salt
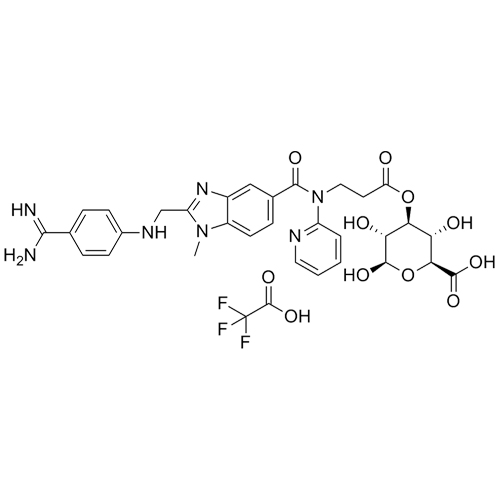
M.F.
M.W. 647.65 114.02
CAT# AR-D01056
CAS# 1296162-15-6 (Free base)
Dabigatran Acyl-O-4-D-Glucuronide Trifluoroacetic Acid Salt
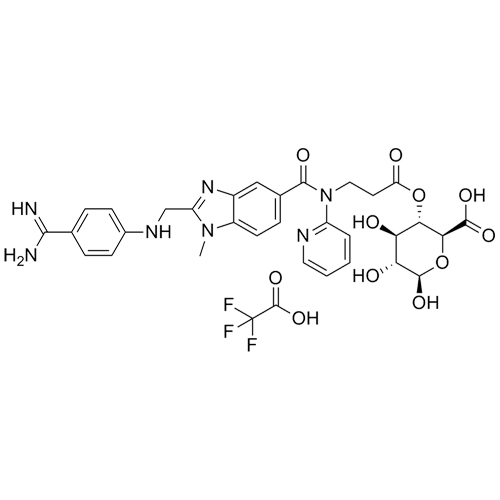
M.F.
M.W. 647.65 114.02
CAT# AR-D01057
CAS# 1296162-17-8 (freebase)
Dabigatran Acyl-O-3-D-Glucuronide Trifluoroacetic Acid Salt

M.F.
M.W. 647.65 114.02
CAT# AR-D06176
CAS# NA
Dabigatran Acyl-O-4-D-Glucuronide Trifluoroacetic Acid Salt
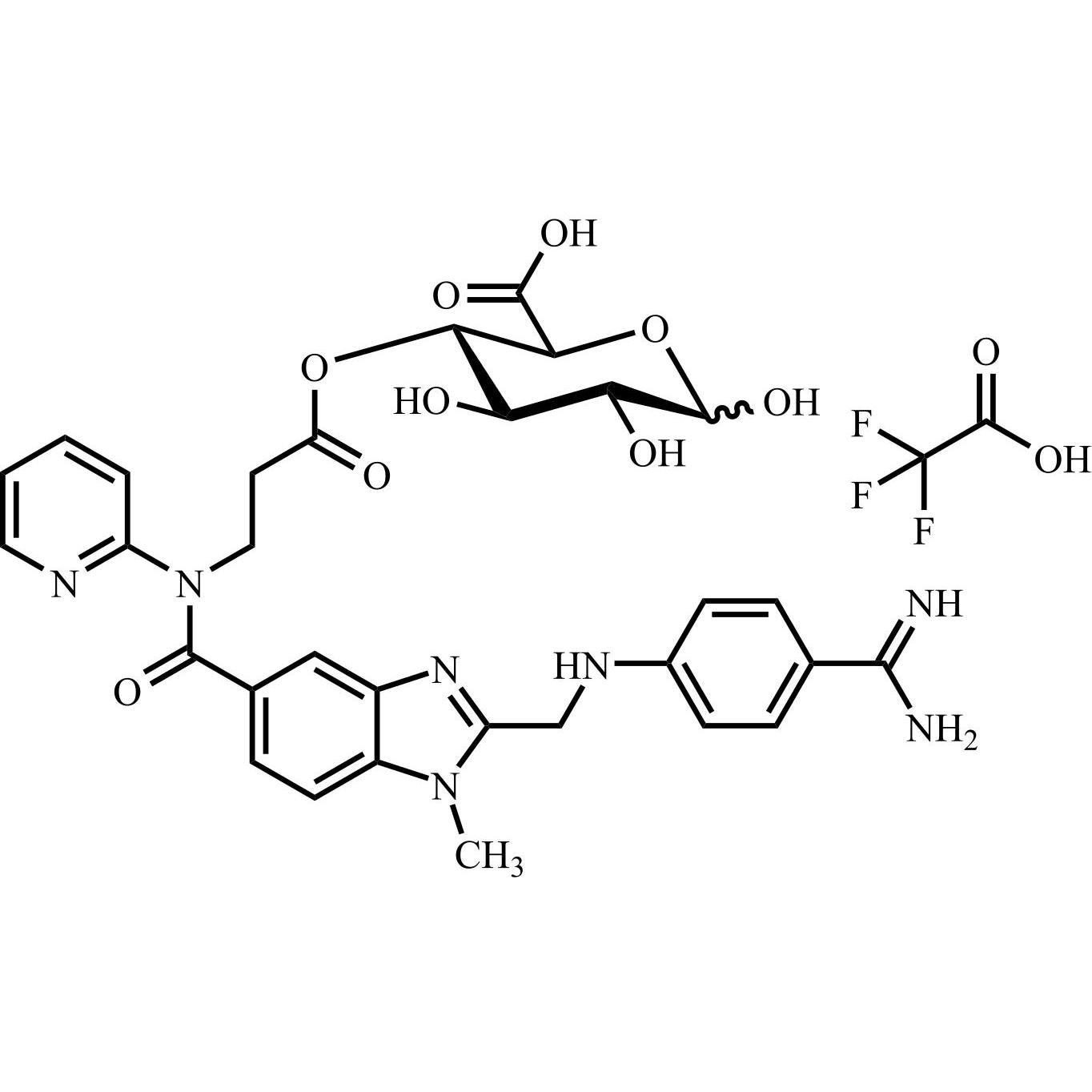
M.F.
M.W. 647.65 114.02
CAT# AR-D06177
CAS# NA
Dabigatran Acyl-O-2-D-Glucuronide Trifluoroacetic Acid Salt

M.F.
M.W. 647.65 114.02
CAT# AR-D06175
CAS# NA