- SynonymsAmbodryl; Bromazin; 2-[(4-bromophenyl)-phenylmethoxy]-N,N-dimethylethanamine; 2-(p-bromo-alpha-phenylbenzyloxy)-N,N-dimethylethylamine; beta-(p-Bromobenzhydryloxy)ethyldimethylamine; Diphenhydramine
- Description
Ambodryl; Bromazin; 2-[(4-bromophenyl)-phenylmethoxy]-N,N-dimethylethanamine; 2-(p-bromo-alpha-phenylbenzyloxy)-N,N-dimethylethylamine; beta-(p-Bromobenzhydryloxy)ethyldimethylamine; Diphenhydramine
Dimenhydrinate EP Impurity C is a fully characterized chemical compound used as a reference standard of API Dimenhydrinate. The standard offered is compliant with regulatory guidelines. Dimenhydrinate EP Impurity C is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 118-23-0
Related products
Dimenhydrinate EP Impurity J (Phenytoin EP Impurity A)
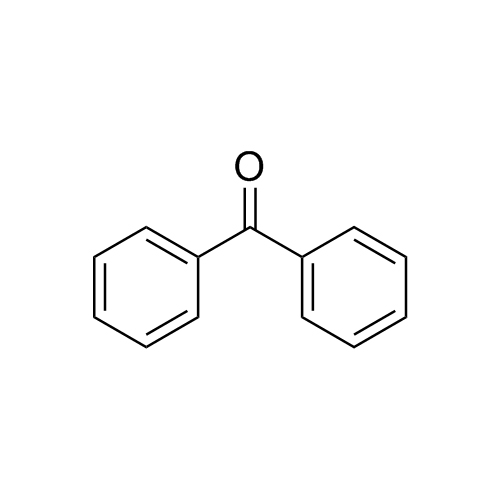
M.F.
M.W. 182.22
CAT# AR-D01879
CAS# 119-61-9
Dimenhydrinate EP Impurity C Hydrochloride salt

M.F.
M.W. 334.3 : 36.5
CAT# AR-D05933
CAS# 1808-12-4
Dimenhydrinate EP Impurity D DiHCl
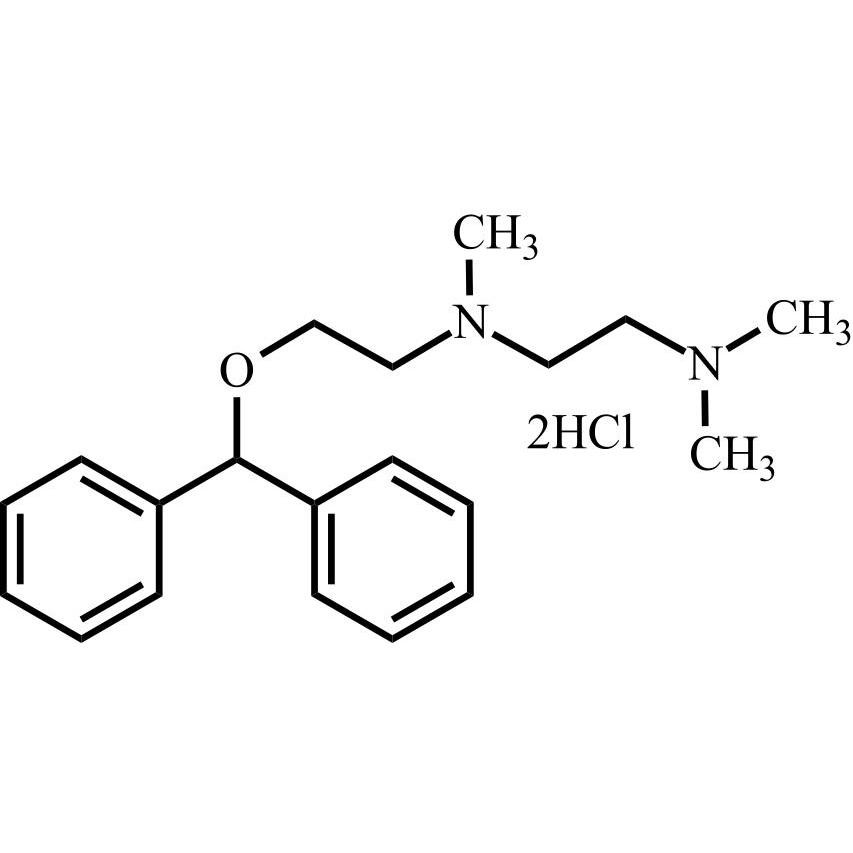
M.F.
M.W. 312.46 2*36.46
CAT# AR-D07116
CAS# 2212-35-3 (free base)















