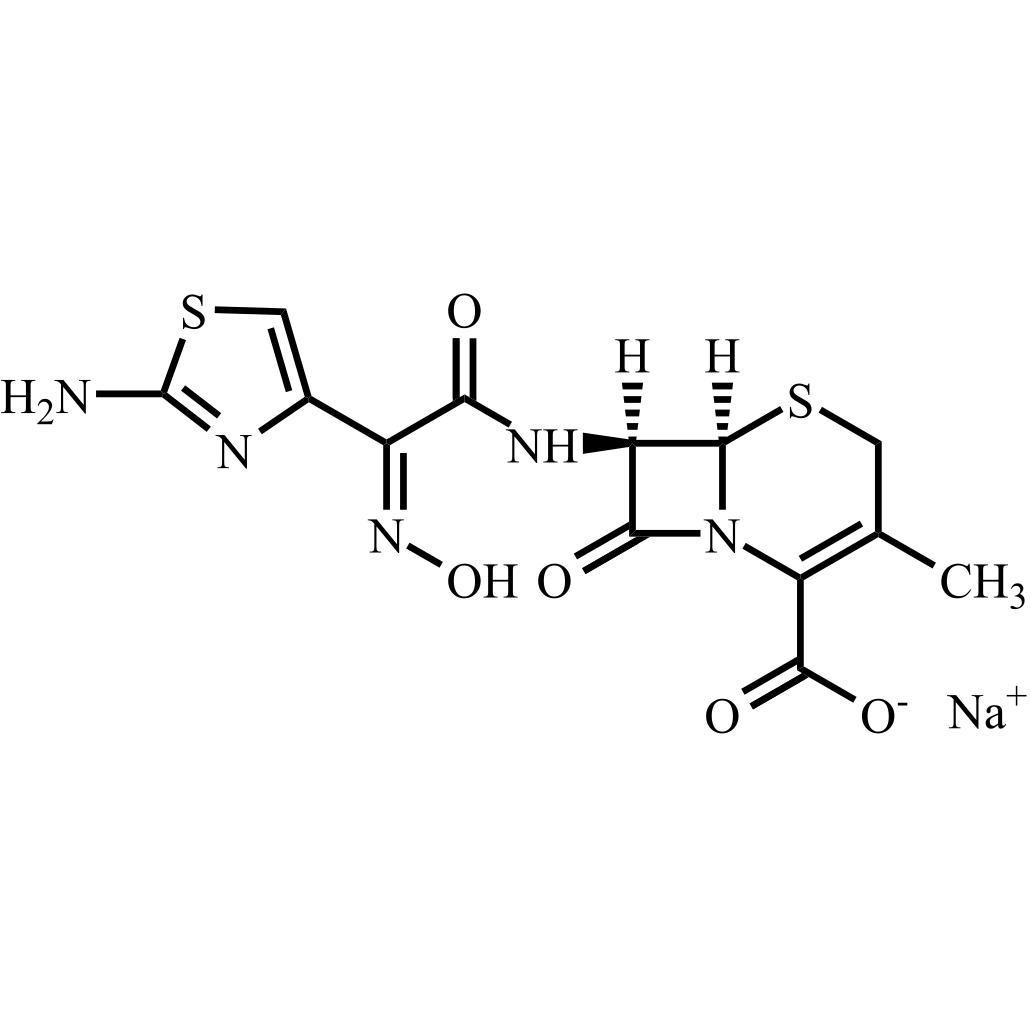
- Synonyms(6R,7R)-7-((E)-2-(2-aminothiazol-4-yl)-2-(hydroxyimino)acetamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2E)-(2-Amino-4-thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid; Cefdinir EP Impurity R
- Description
(6R,7R)-7-((E)-2-(2-aminothiazol-4-yl)-2-(hydroxyimino)acetamido)-8-oxo-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid; (6R,7R)-7-[[(2E)-(2-Amino-4-thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid; Cefdinir EP Impurity R
(E)-Cefdinir is a fully characterized chemical compound used as a reference standard of API Cefdinir. The standard offered is compliant with regulatory guidelines. (E)-Cefdinir is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 178601-88-2
Related products
Cefdinir Decarboxy Open Ring Lactone (Mixture of Diastereomers)

M.F.
M.W. 369.42
CAT# AR-C01753
CAS# 178949-04-7
Cefdinir USP Related Compound A (Mixture of Diastereomers)

M.F.
M.W. 413.42
CAT# AR-C05388
CAS# 178422-42-9
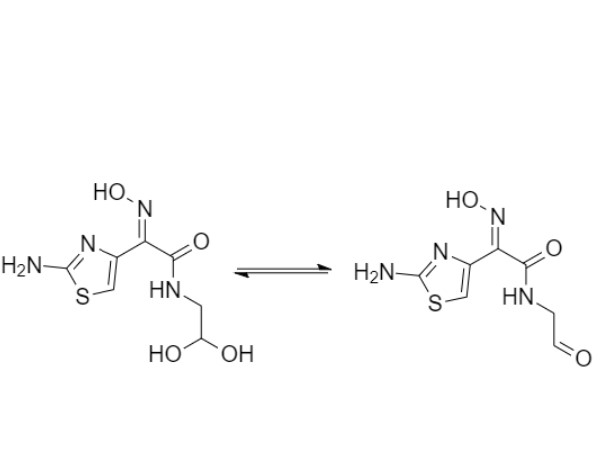
Cefdinir Impurity 6 (Thiazolylacetylglycine Oxime Acetal)

M.F.
M.W. 246.24 228.23
CAT# AR-C06565
CAS# NA