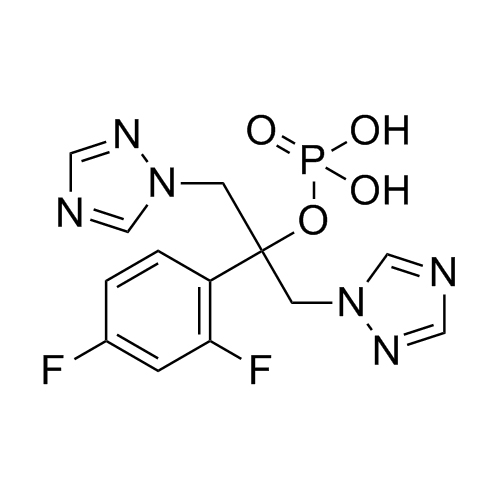
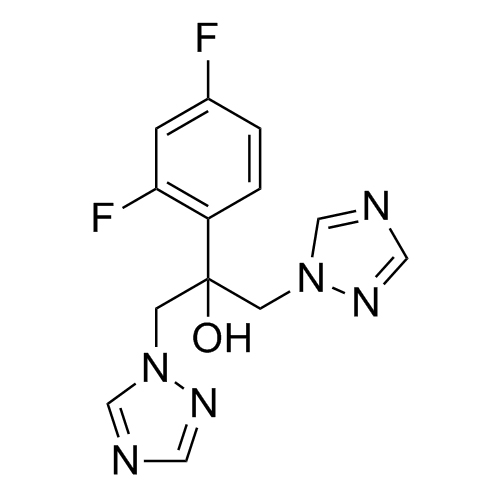
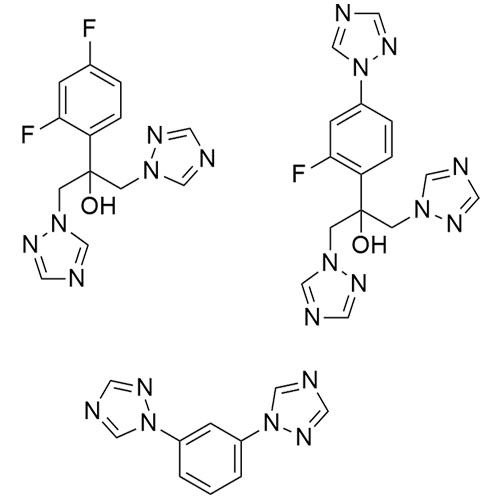
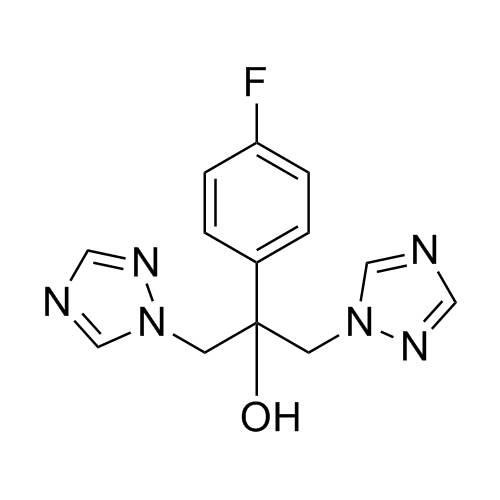
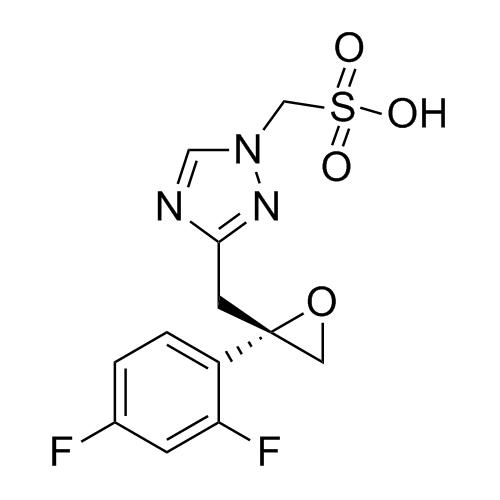
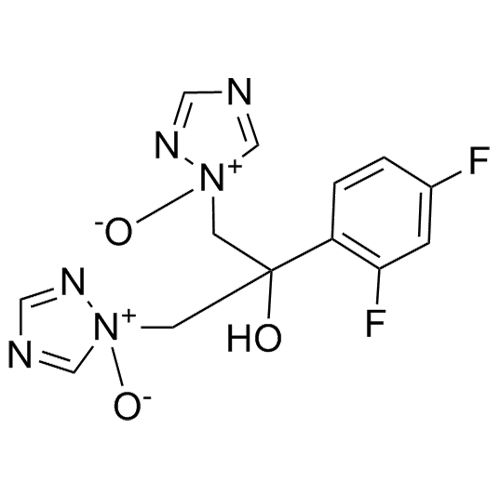
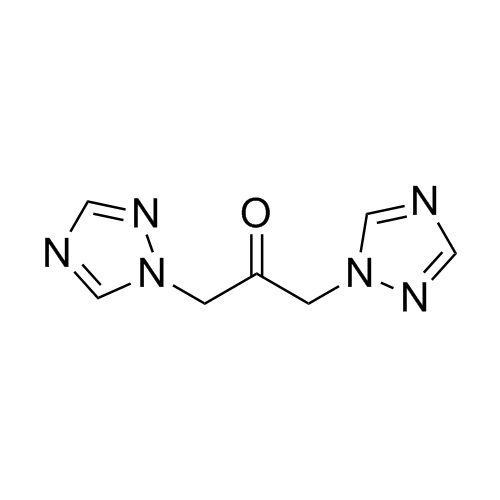
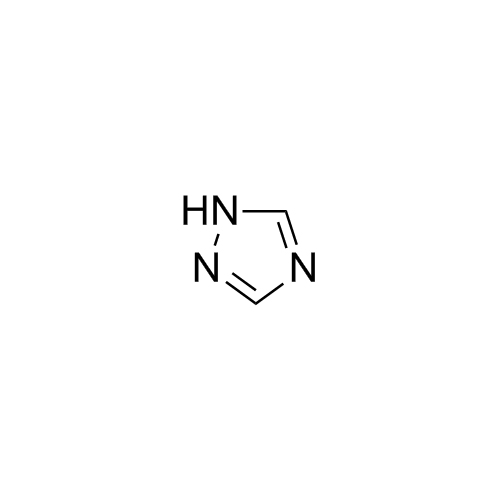
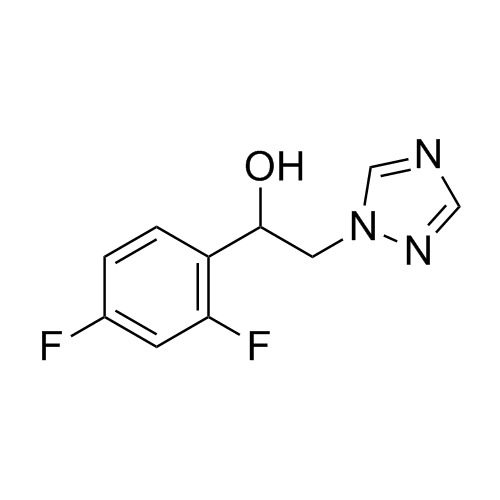
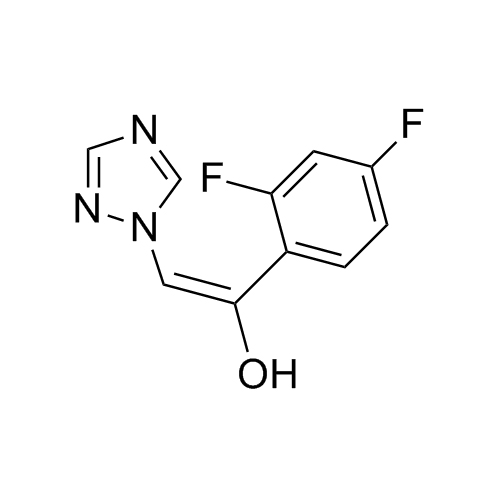
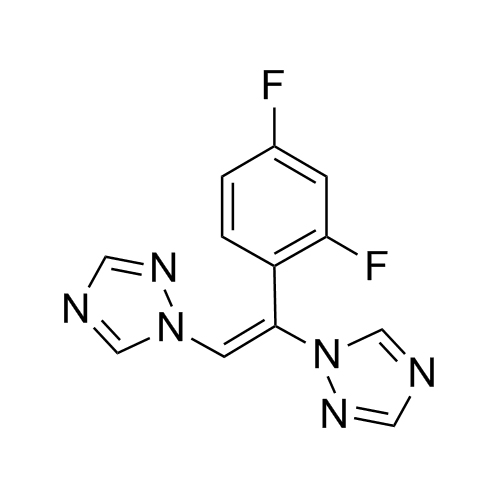
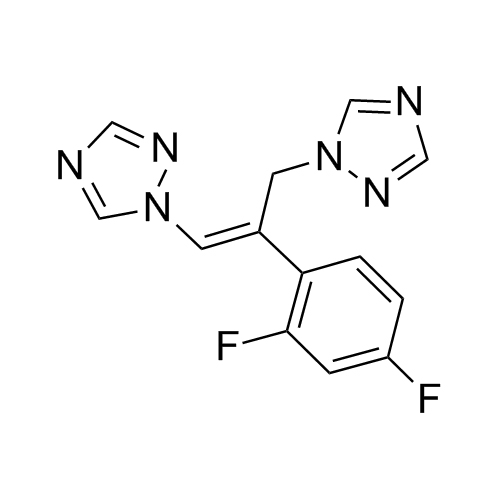
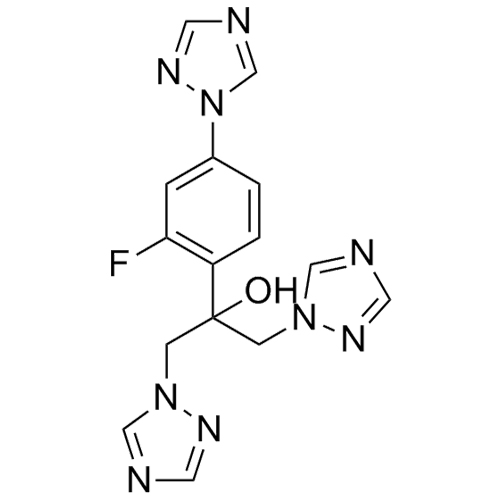
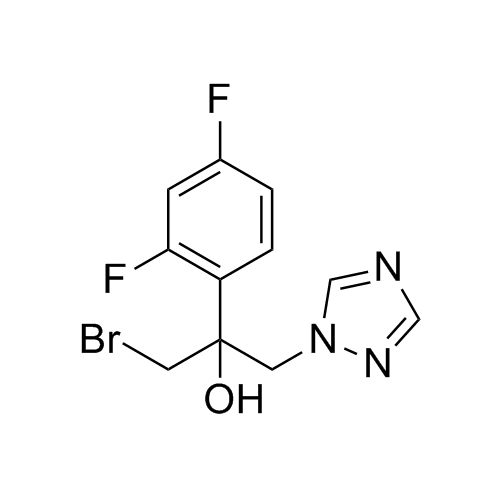
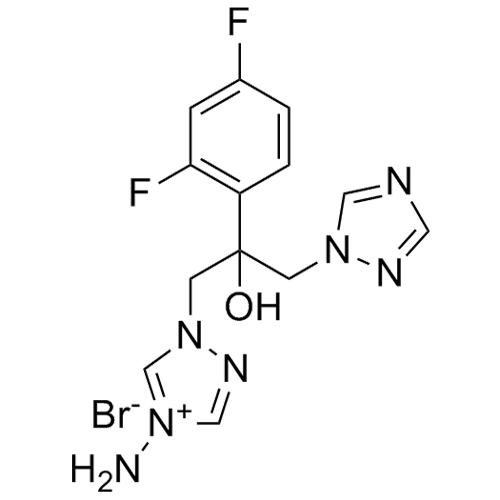
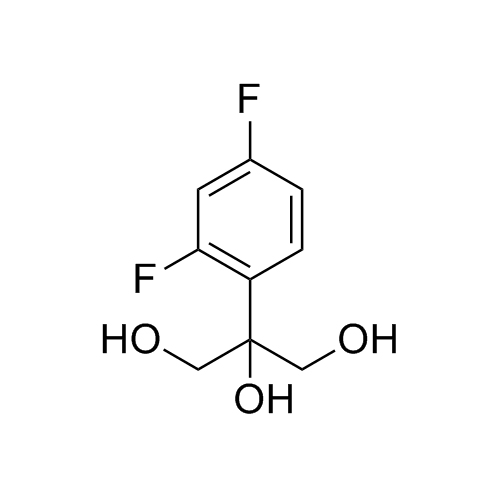
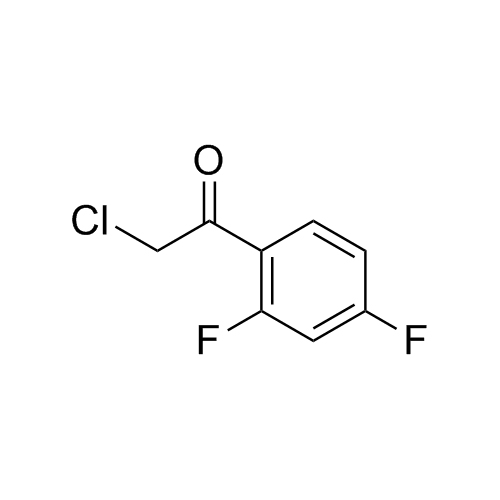
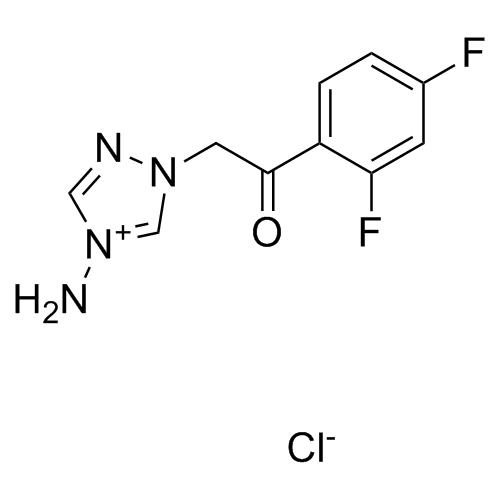
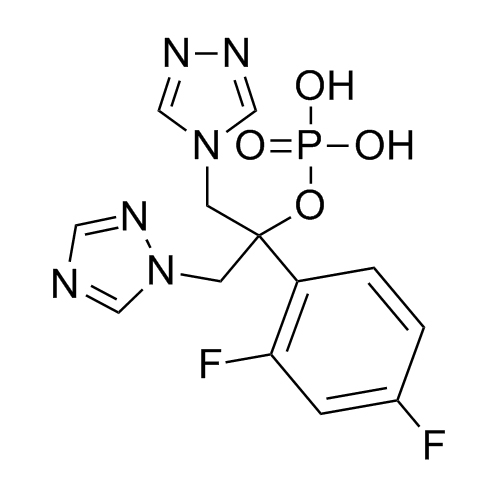
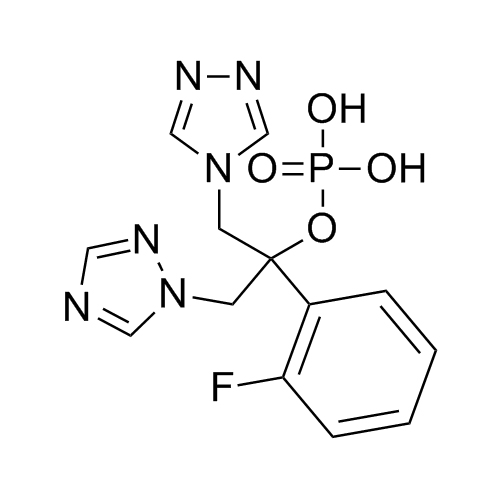
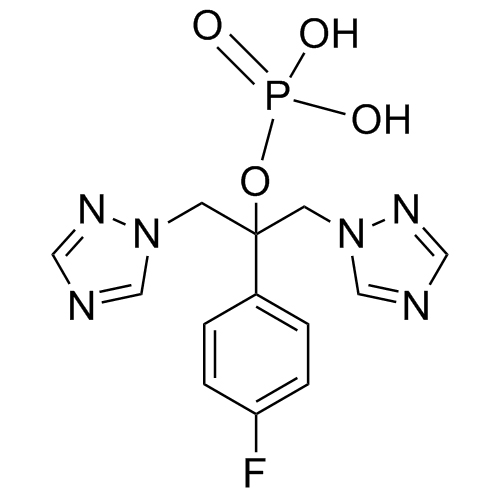
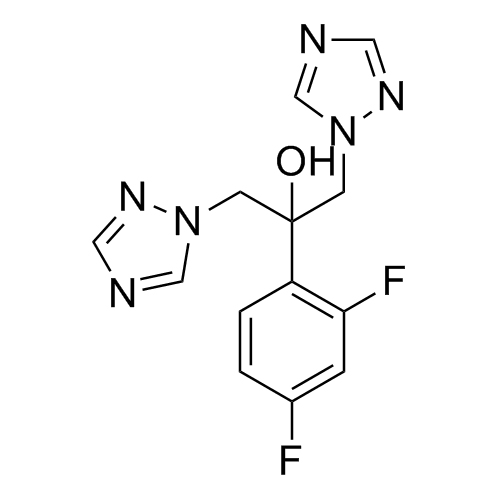
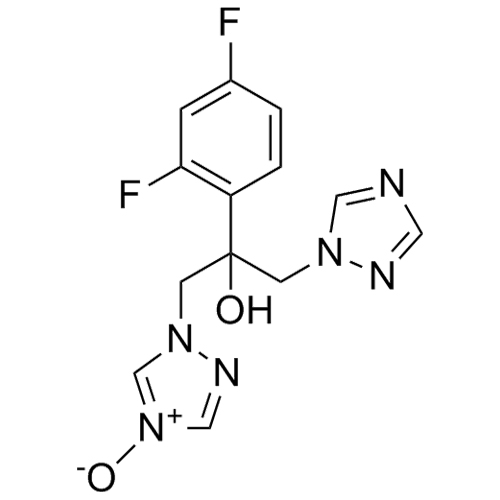
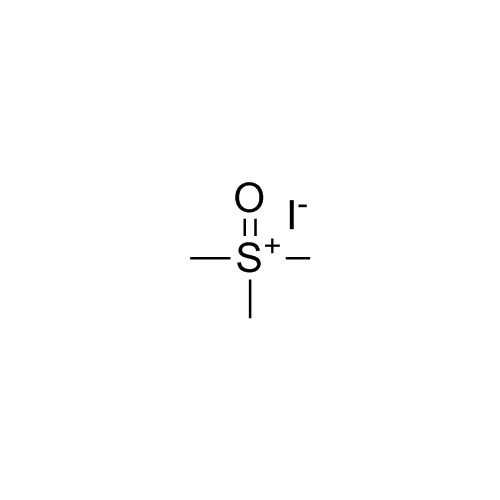
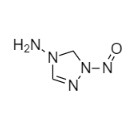
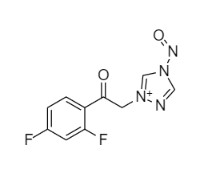
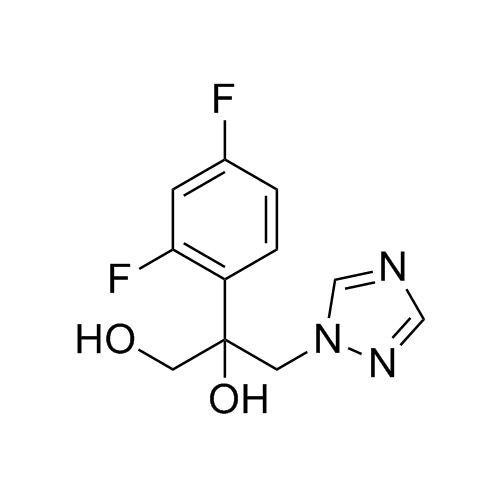
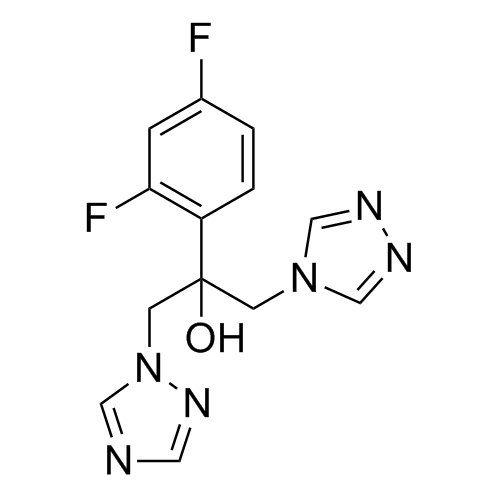
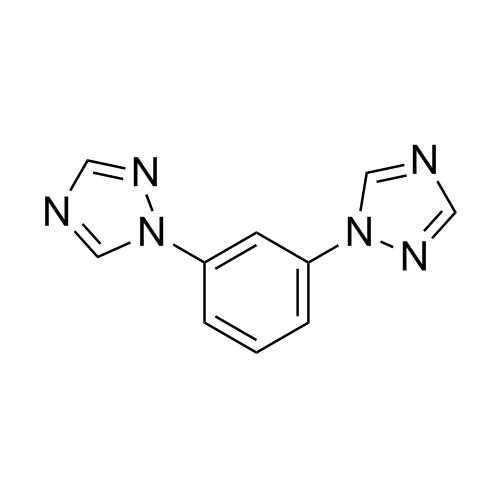
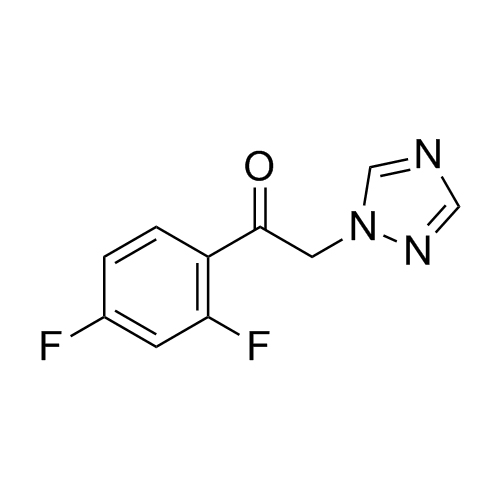
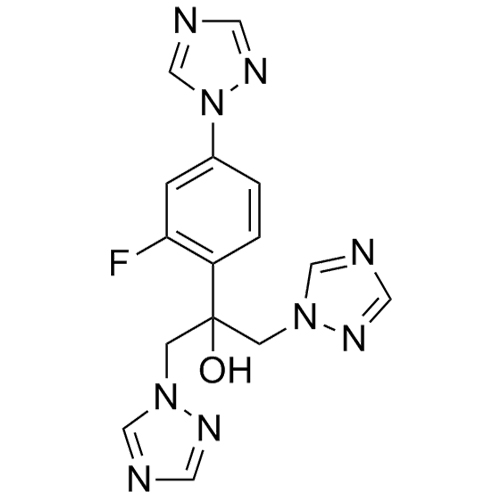
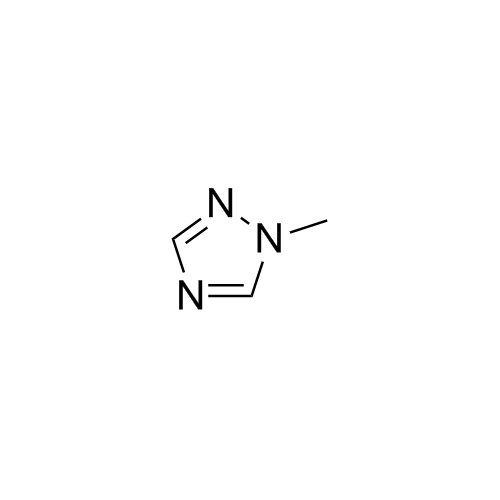
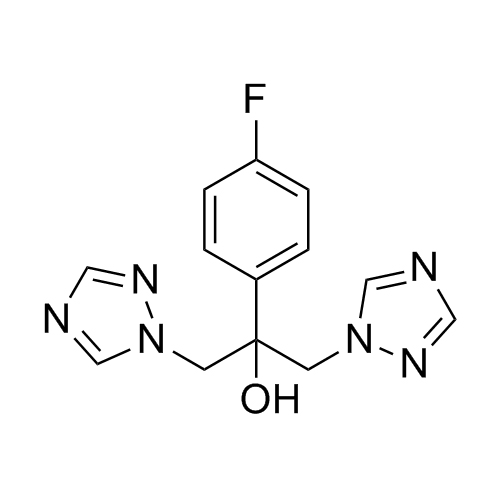
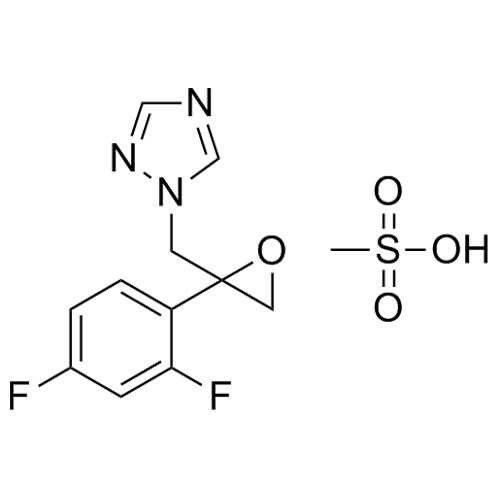
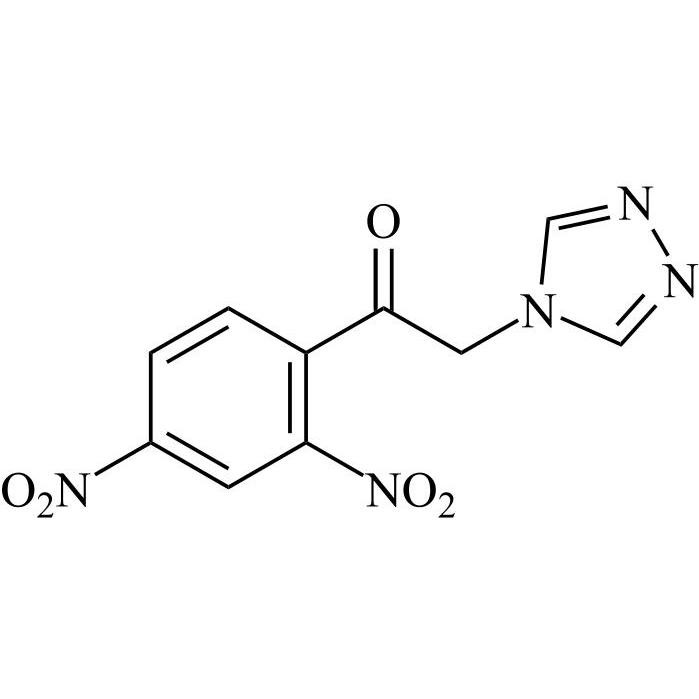
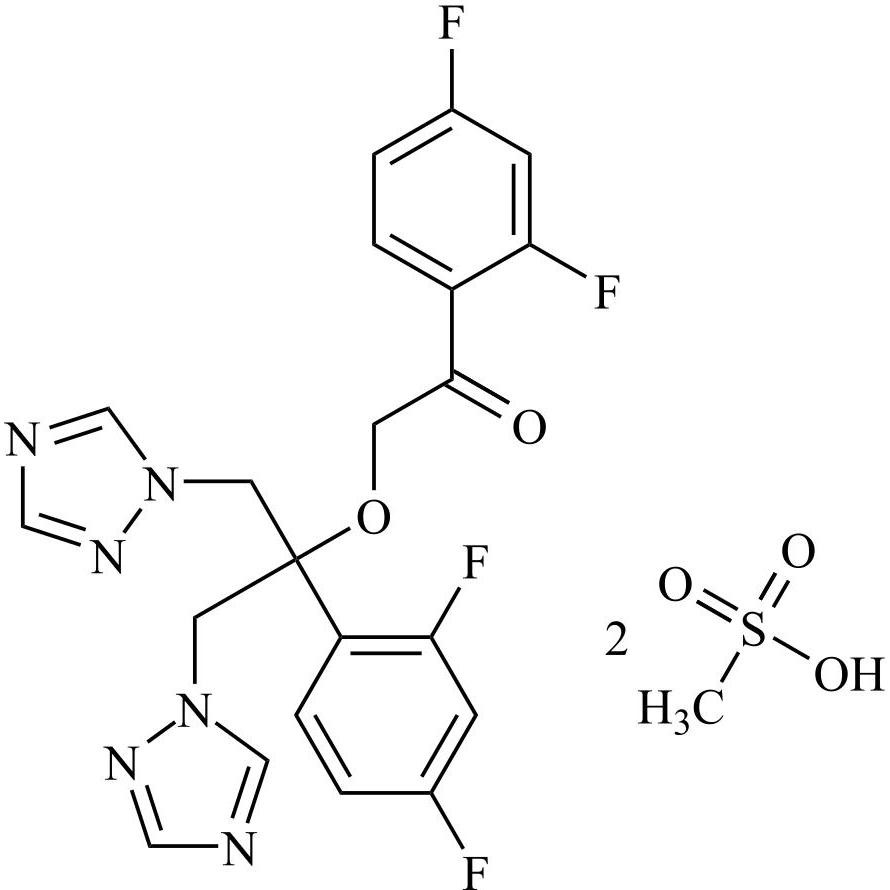
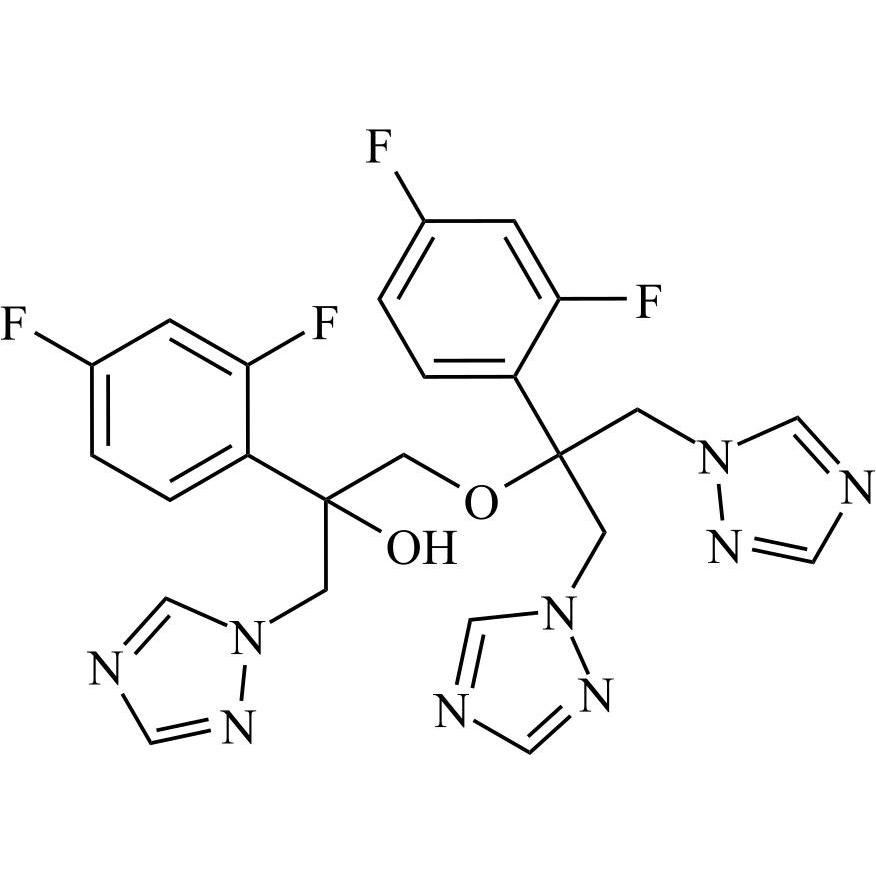
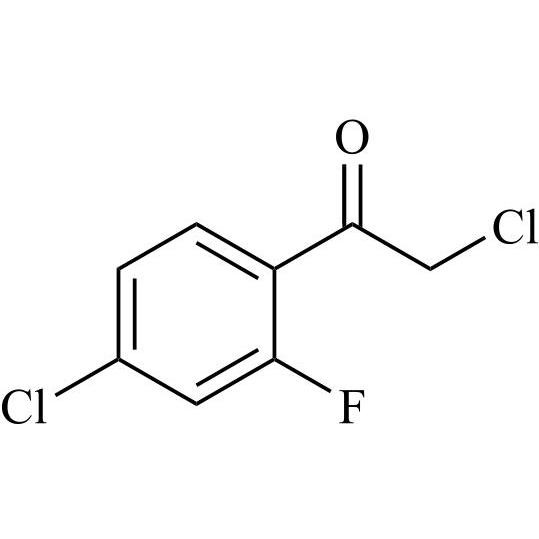
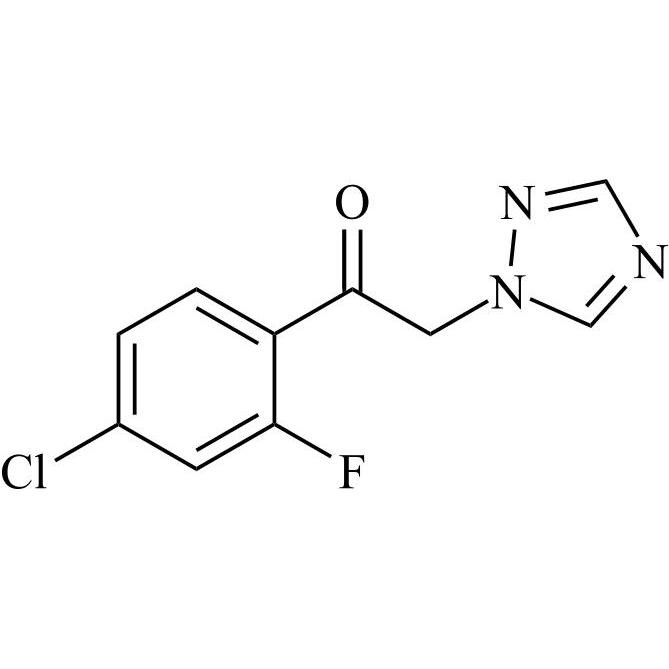
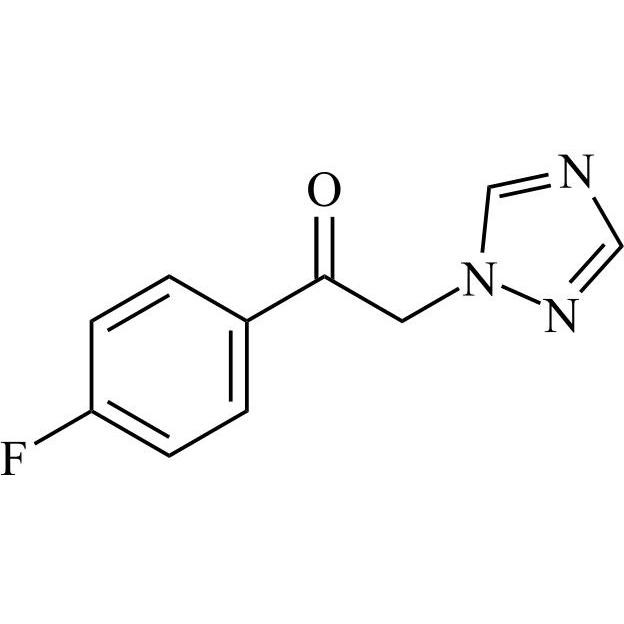
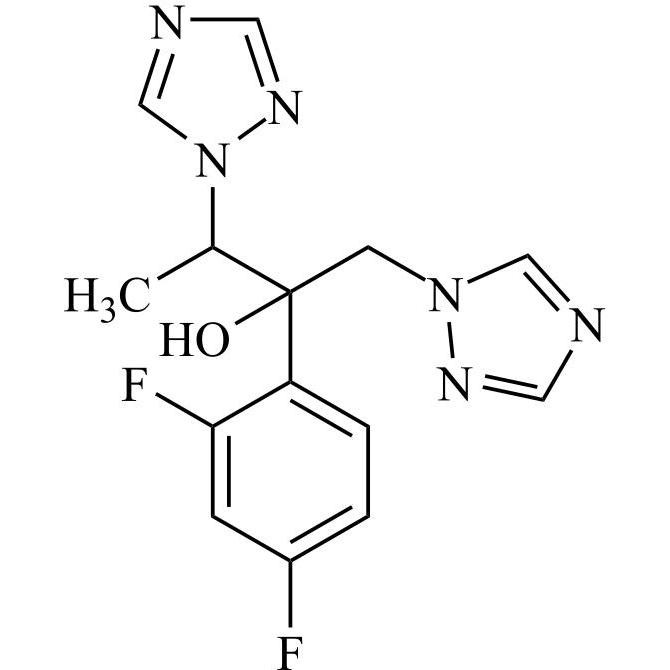
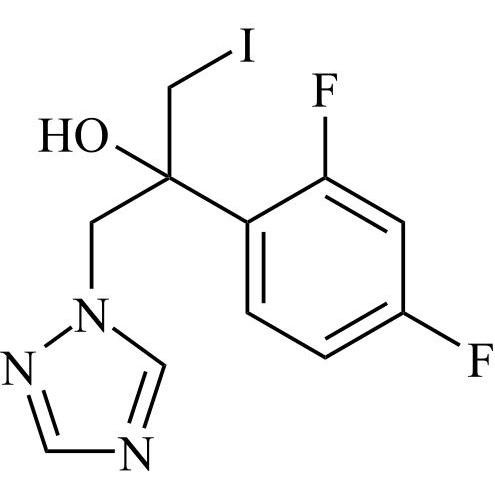
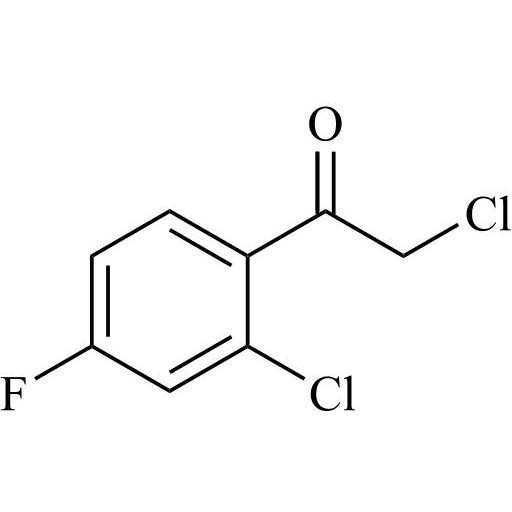
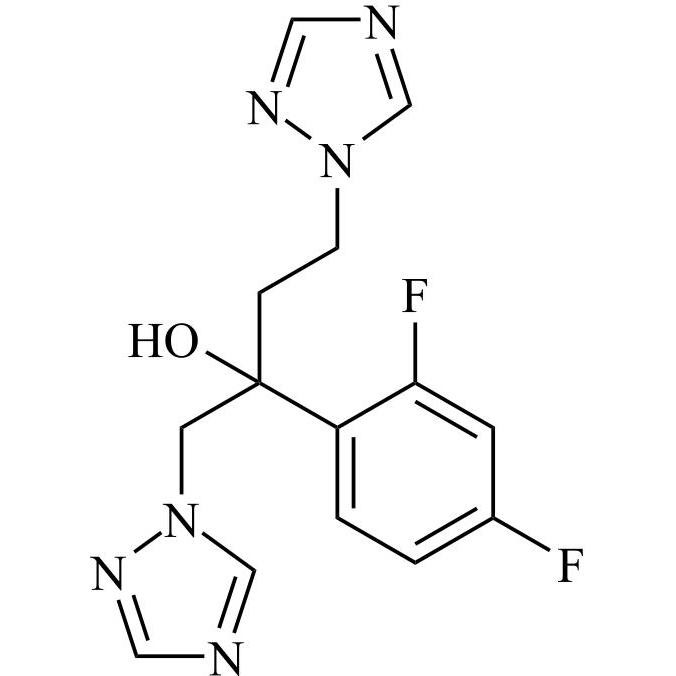
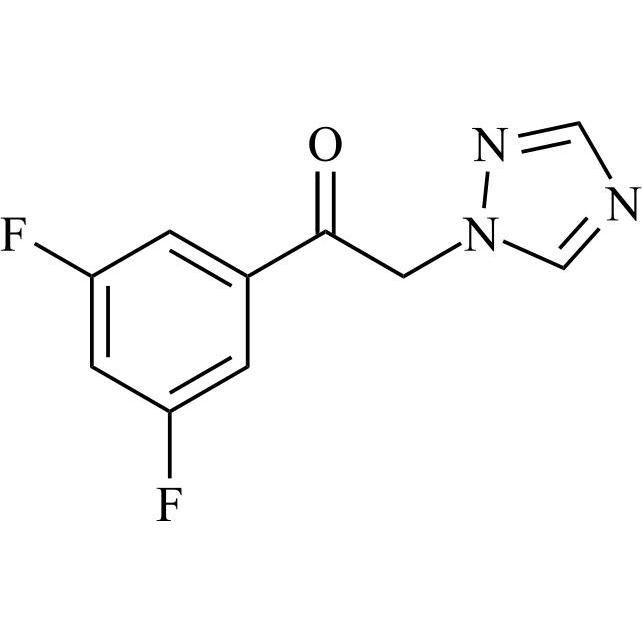
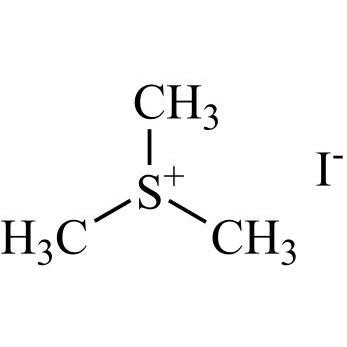
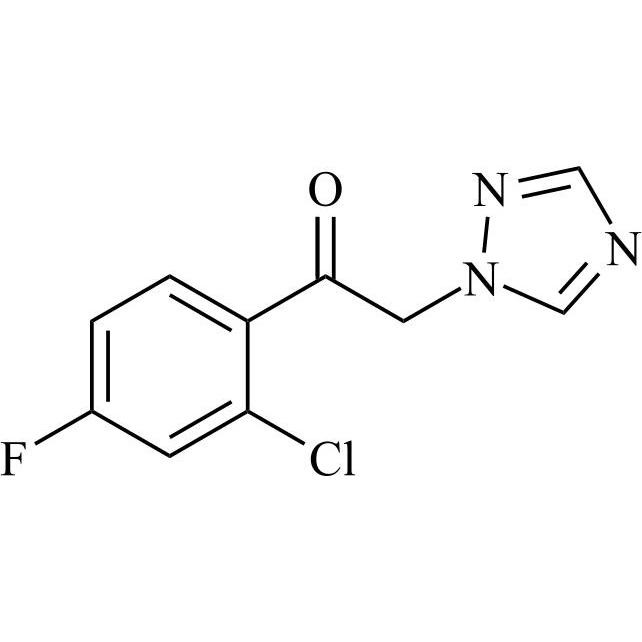
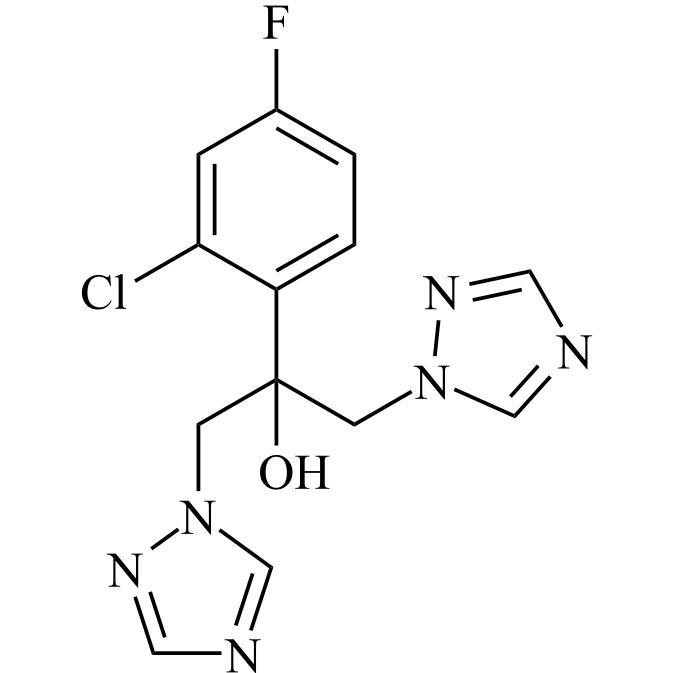
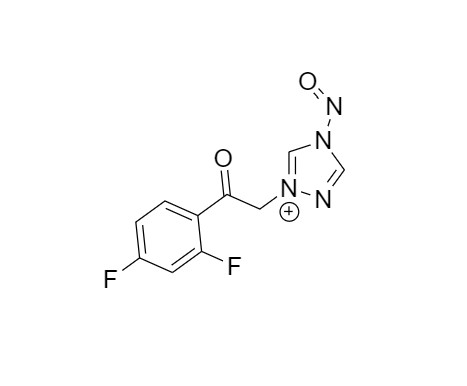
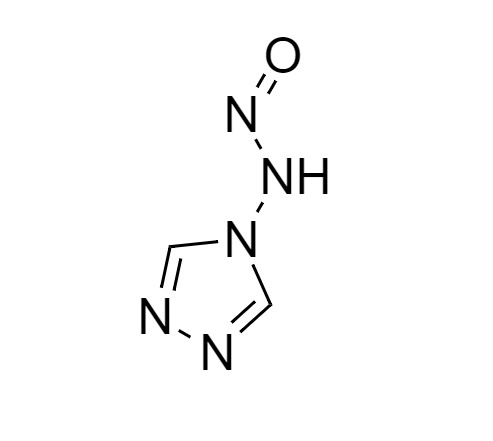
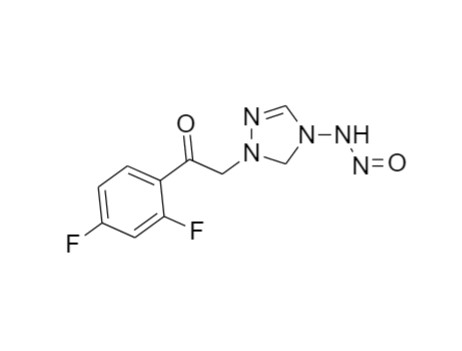
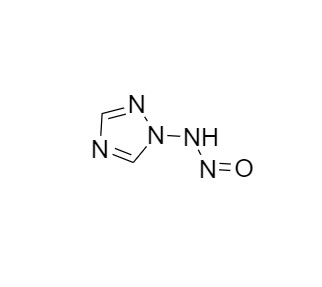
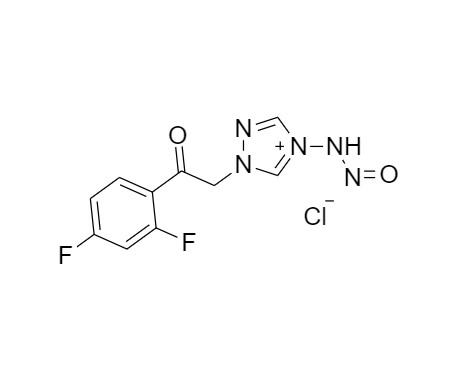
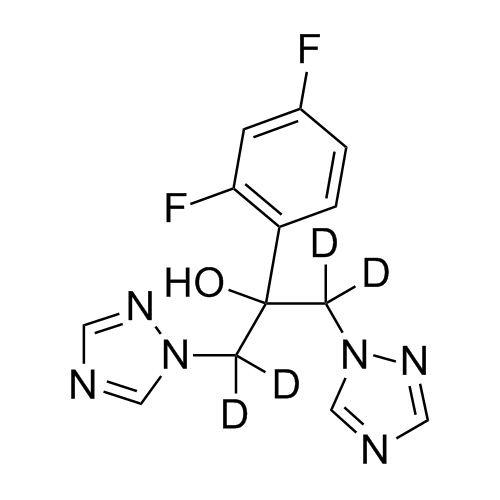
2-[[(2,4-Difluorophenyl)-2-oxiranyl]methyl]-1H-1,2,4-triazole
Fluconazole EP Impurity G is a fully characterized chemical compound used as a reference standard of API Fluconazole. The standard offered is compliant with regulatory guidelines. Fluconazole EP Impurity G is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 86386-76-7