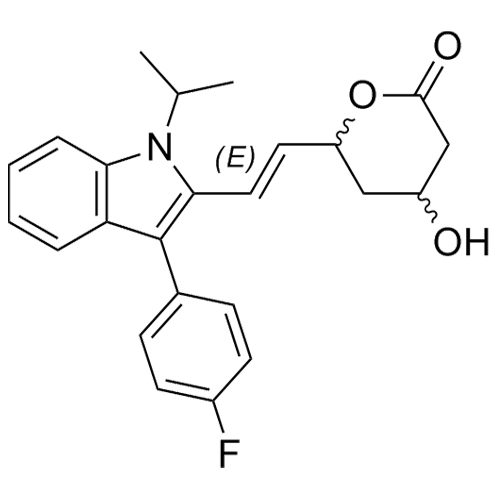
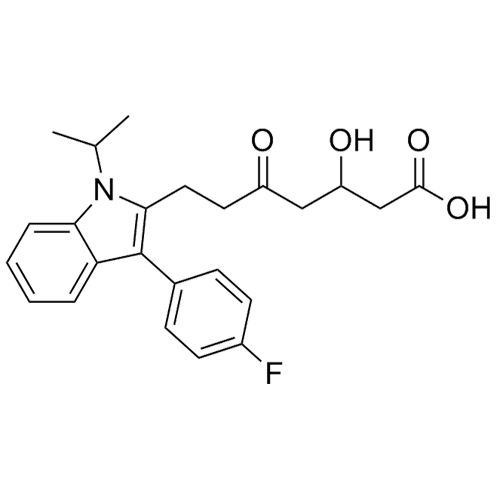
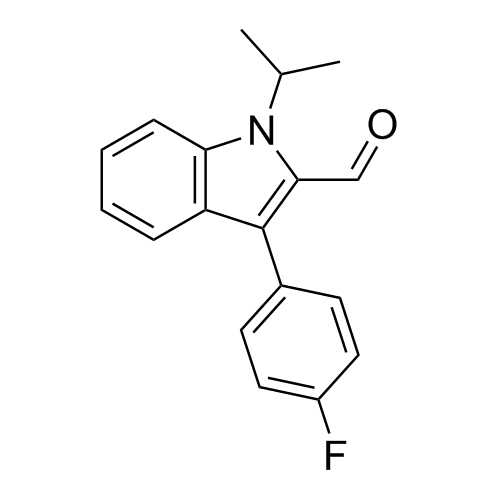
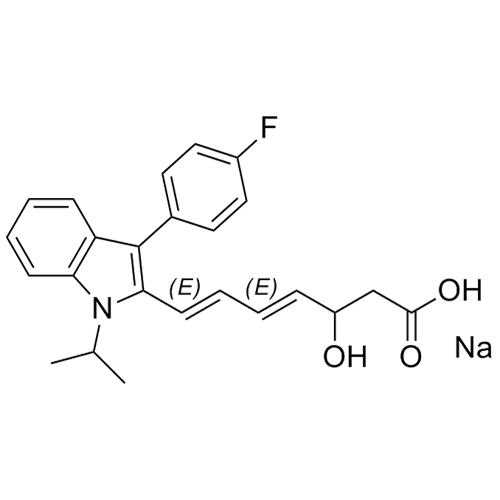
- Synonyms(E)-7-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)-3-hydroxy-5-oxohept-6-enoicacid;(E)-(+/-)-7-[3-(4-Fluorophenyl)-1-(methylethyl)-1H-indol-2-yl]-3-hydroxy-5-oxo-6-heptenoic Acid; Fluvastatin Impurity; Fluvastatin EP Impurity D;rac 5-Keto Fluvastatin
- Description
(E)-7-(3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl)-3-hydroxy-5-oxohept-6-enoicacid;(E)-(+/-)-7-[3-(4-Fluorophenyl)-1-(methylethyl)-1H-indol-2-yl]-3-hydroxy-5-oxo-6-heptenoic Acid; Fluvastatin Impurity; Fluvastatin EP Impurity D;rac 5-Keto Fluvastatin
Fluvastatin EP Impurity D is a fully characterized chemical compound used as a reference standard of API Fluvastatin. The standard offered is compliant with regulatory guidelines. Fluvastatin EP Impurity D is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 1160169-39-0
Related products
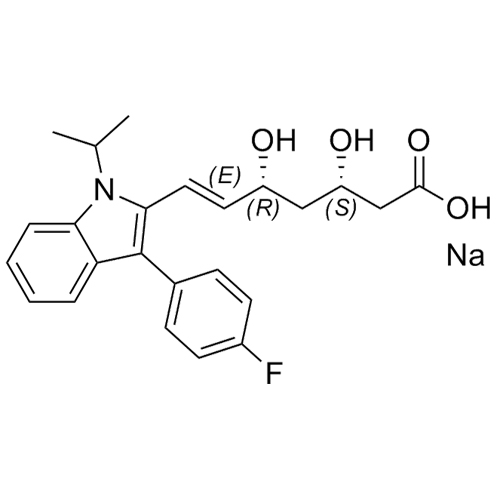
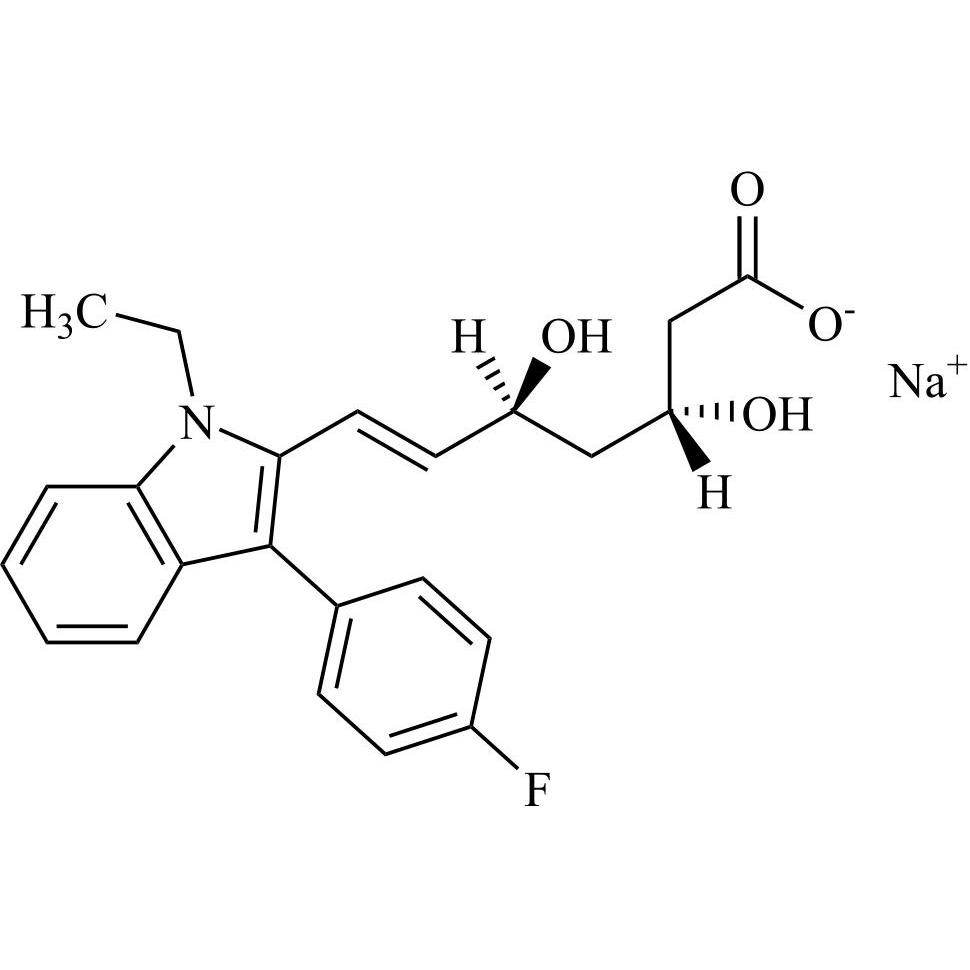
(3S,5R)-Fluvastatin-d6 Sodium Salt

M.F.
M.W. 416.51 22.99
CAT# AR-F01901
CAS# 94061-81-1 (non-labelled)
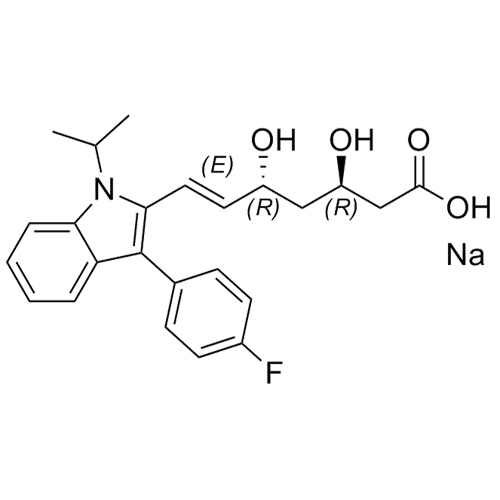
(3R,5S)-Fluvastatin-d6 Sodium Salt

M.F.
M.W. 416.51 22.99
CAT# AR-F01902
CAS# 94061-80-0 (non-labelled)