- Synonyms(1R,2S,3R)-1-(5-((2S,3R)-2,3,4-trihydroxybutyl)pyrazin-2-yl)butane-1,2,3,4-tetraol; 2,5-DEOXYFRUCTOSAZINE; (1R,2S,3R)-1-[5-[(2S,3R)-2,3,4-Trihydroxybutyl]-2-pyrazinyl]-1,2,3,4-butanetetrol; 2-(D-arabino-Tetrahydroxybutyl)-5-(D-erythro-2,3,4-trihydroxybutyl)pyrazine; NSC 270912; (Deoxy-Fructosazine)
- Description
(1R,2S,3R)-1-(5-((2S,3R)-2,3,4-trihydroxybutyl)pyrazin-2-yl)butane-1,2,3,4-tetraol; 2,5-DEOXYFRUCTOSAZINE; (1R,2S,3R)-1-[5-[(2S,3R)-2,3,4-Trihydroxybutyl]-2-pyrazinyl]-1,2,3,4-butanetetrol; 2-(D-arabino-Tetrahydroxybutyl)-5-(D-erythro-2,3,4-trihydroxybutyl)pyrazine; NSC 270912; (Deoxy-Fructosazine)
Glucosamine EP Impurity C is a fully characterized chemical compound used as a reference standard of API Glucosamine. The standard offered is compliant with regulatory guidelines. Glucosamine EP Impurity C is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 17460-13-8
Related products
Glucosamine Sulfate Potassium Chloride

M.F.
M.W. 2(179.17); 98.07; 2(74.55)
CAT# AR-M52645
CAS# 1296149-08-0
Benzyl 2-Acetamido-4,6-O-Benzylidene-2-Deoxy-a-D-Muramic Acid Methyl Ester
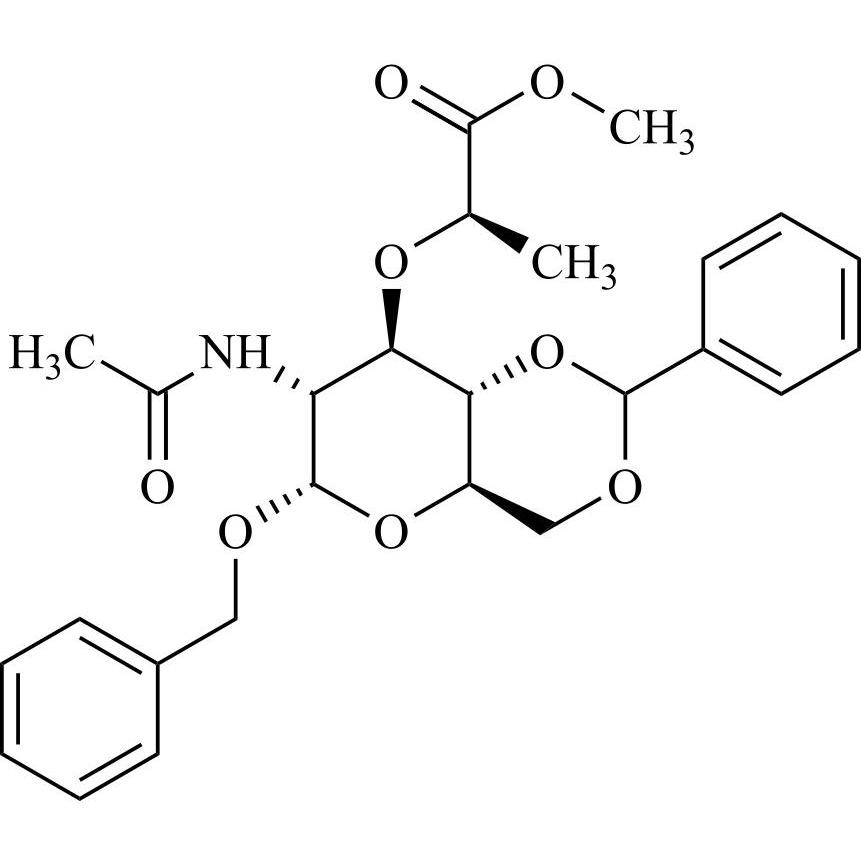
M.F.
M.W. 485.53
CAT# AR-G02369
CAS# 104371-51-9
N-Acetylglucosamine 4-Sulfate Triethylamine Salt (Mixture of Diastereomers)

M.F.
M.W. 301.27 101.19
CAT# AR-G02374
CAS# NA
N-Acetyl-4-O-[2-(acetylamino)-2-deoxy-ß-D-glucopyranosyl]muramic Acid
![Show details for N-Acetyl-4-O-[2-(acetylamino)-2-deoxy-ß-D-glucopyranosyl]muramic Acid Picture of N-Acetyl-4-O-[2-(acetylamino)-2-deoxy-ß-D-glucopyranosyl]muramic Acid](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-G02367.jpg?size=256)
M.F.
M.W. 496.47
CAT# AR-G02367
CAS# 41137-10-4
2-Amino-6-O-(2-amino-2-deoxy-a-D-glucopyranosyl)-2-deoxy-D-glucose 2HCl
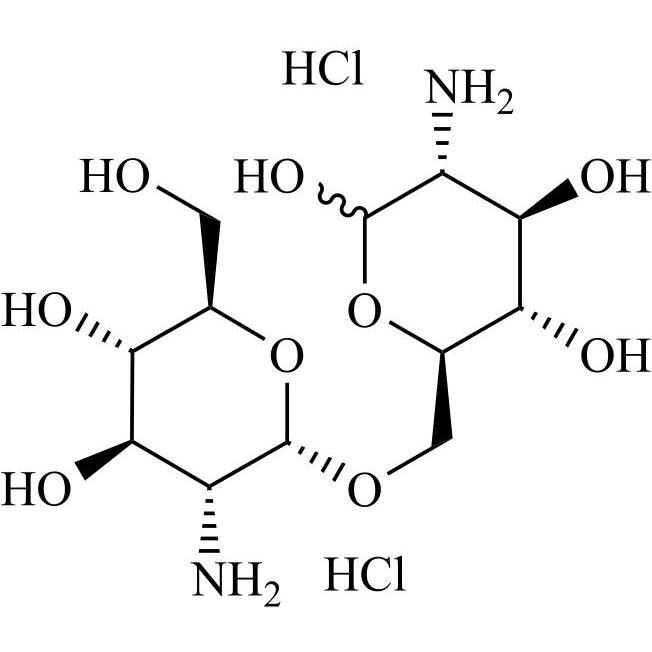
M.F.
M.W. 340.33 2*36.46
CAT# AR-G02366
CAS# 67546-27-4

















