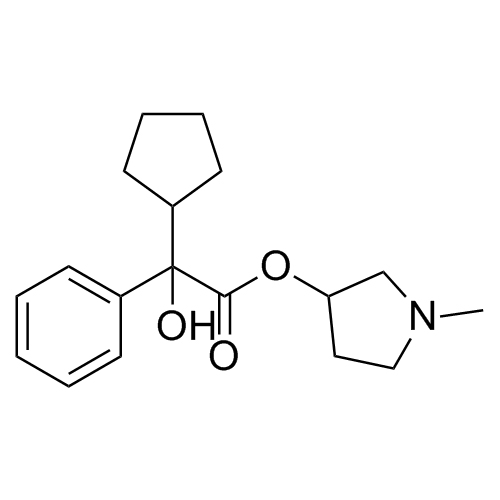
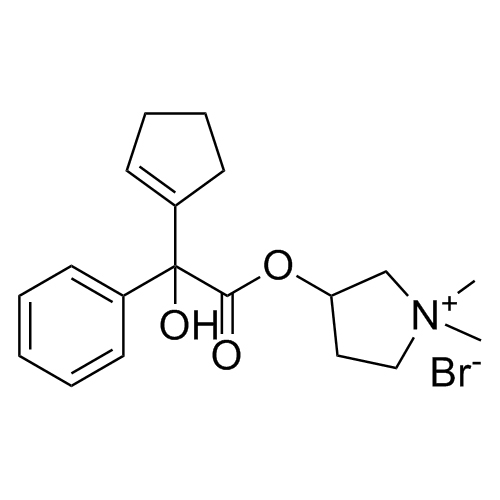
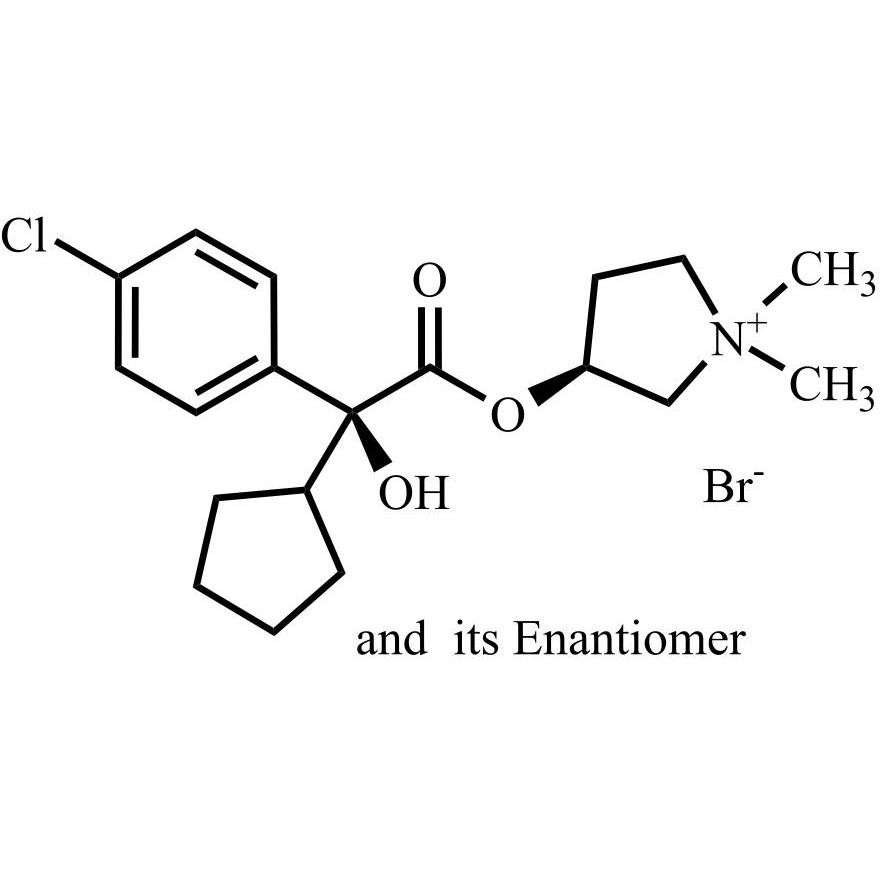
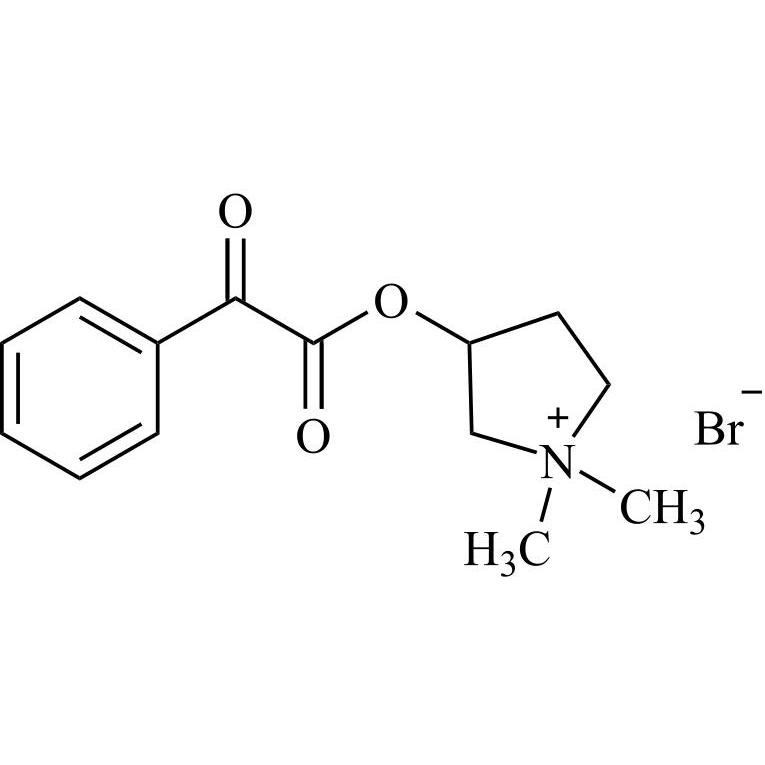
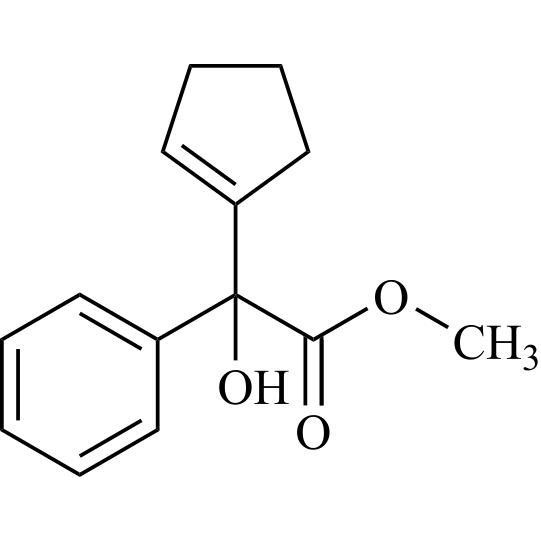
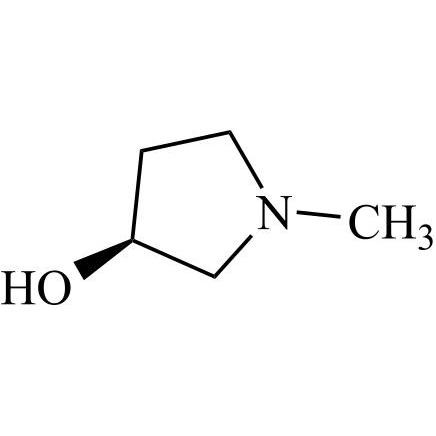
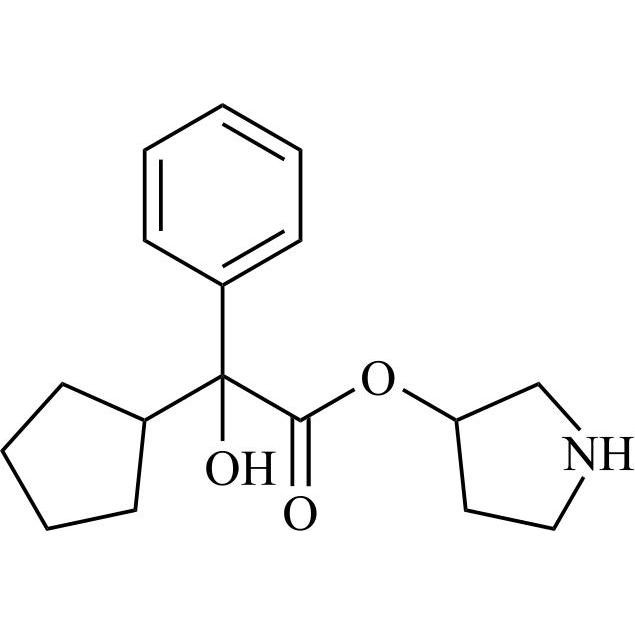
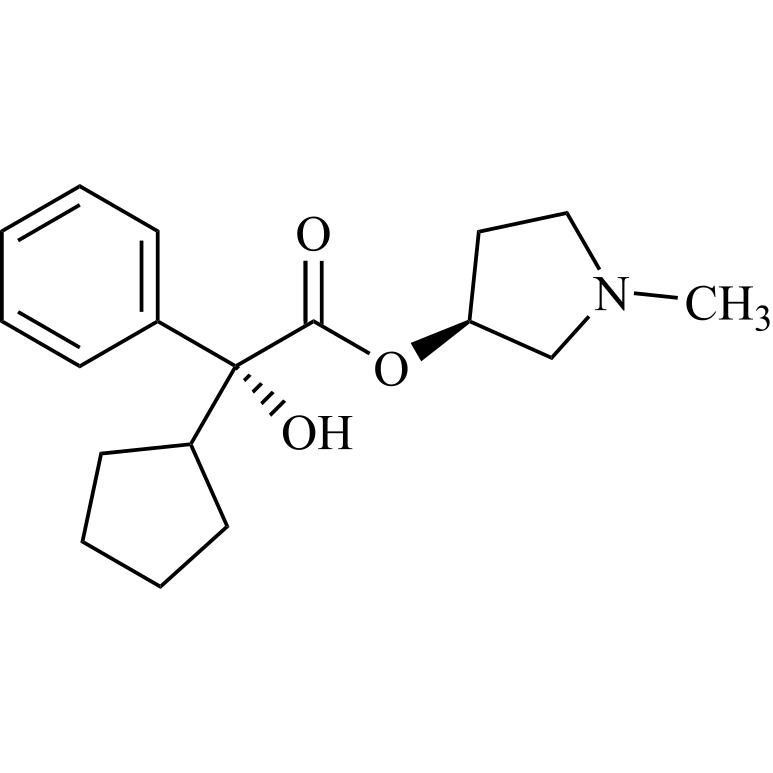
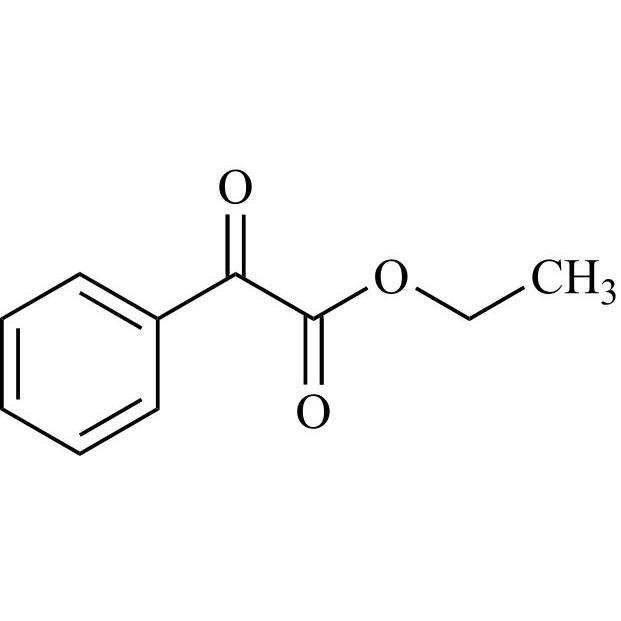
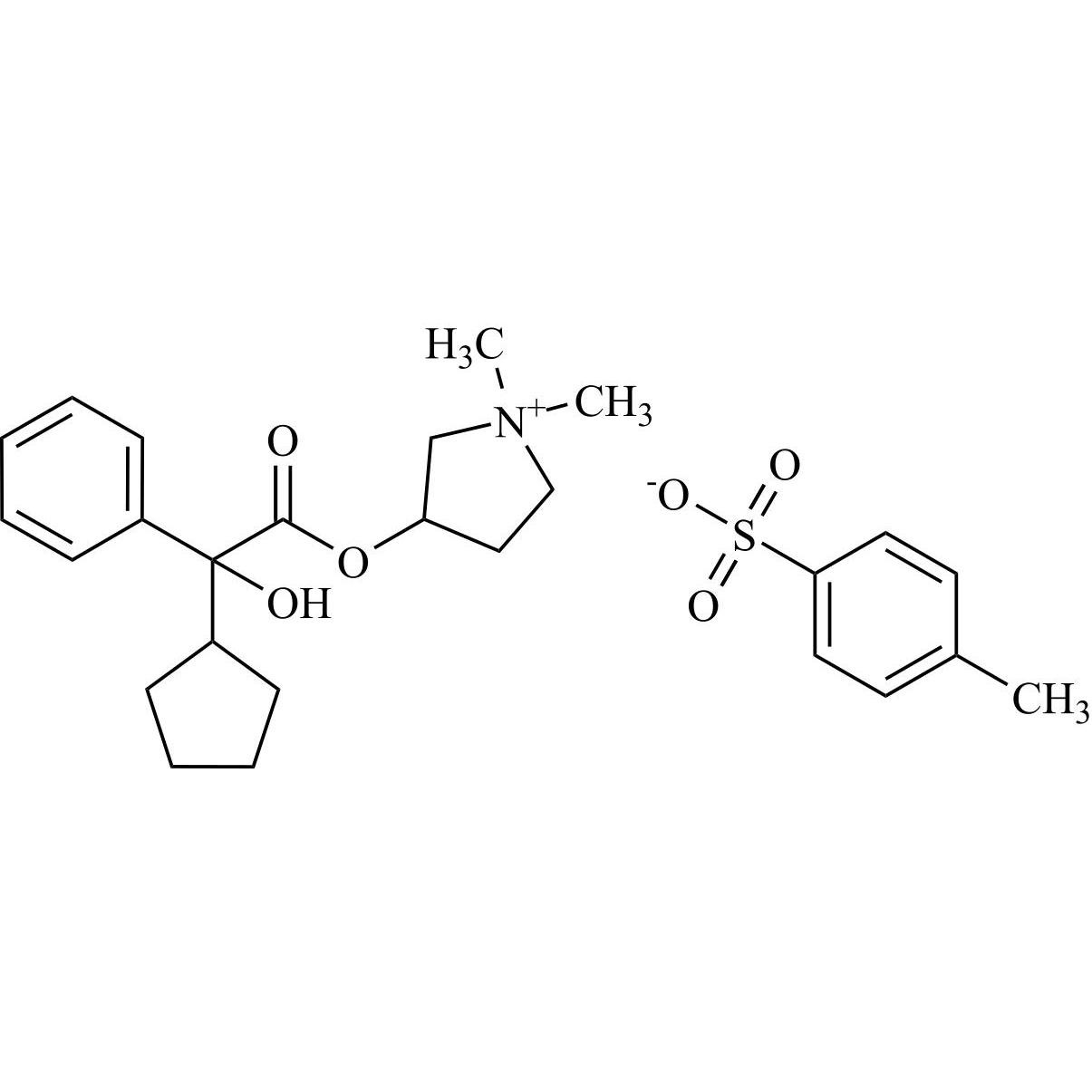
- Synonymsmethyl2-cyclopentyl-2-hydroxy-2-phenylacetate;?-Cyclopentyl-?-hydroxy-benzeneacetic Acid Methyl Ester; ?-Cyclopentyl-mandelic Acid Methyl Ester; Methyl Cyclopentylphenylglycolate; Methyl ?-Cyclopentylmandelate
- Description
methyl2-cyclopentyl-2-hydroxy-2-phenylacetate;?-Cyclopentyl-?-hydroxy-benzeneacetic Acid Methyl Ester; ?-Cyclopentyl-mandelic Acid Methyl Ester; Methyl Cyclopentylphenylglycolate; Methyl ?-Cyclopentylmandelate
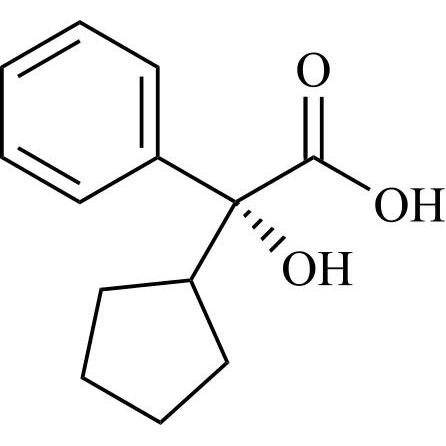
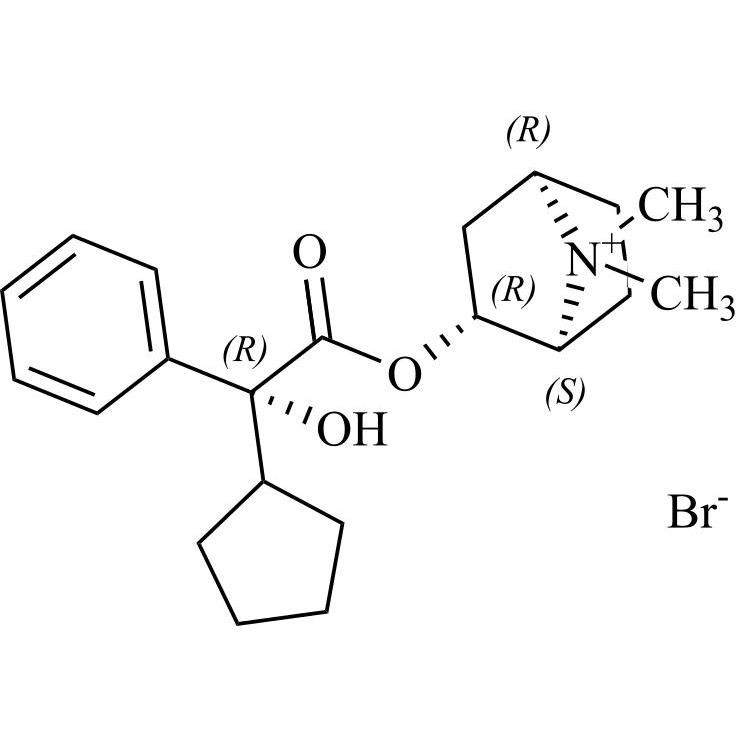
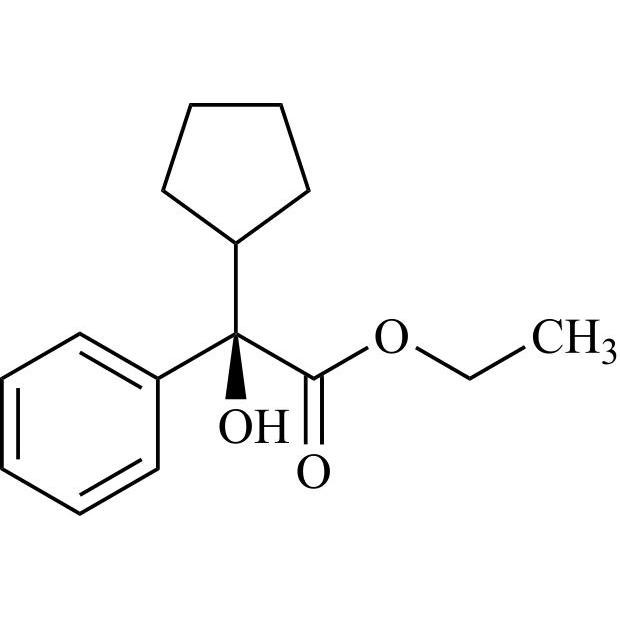
Glycopyrrolate EP Impurity L is a fully characterized chemical compound used as a reference standard of API Glycopyrrolate. The standard offered is compliant with regulatory guidelines. Glycopyrrolate EP Impurity L is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 19833-96-6
Related products
Glycopyrrolate Impurity I (mixture of RR-Isomer and SS-Isomer)

M.F.
M.W. 352.88 79.90
CAT# AR-G01564
CAS# NA
Glycopyrrolate Impurity I (Mixture of RS-Isomer and SR-Isomer)

M.F.
M.W. 352.88 79.90
CAT# AR-G01565
CAS# NA
Glycopyrrolate Erythro Isomer (Mixture of RR-Isomer and SS-Isomer)

M.F.
M.W. 318.44 79.90
CAT# AR-G01567
CAS# 58493-54-2
Glycopyrronium Bromide EP Impurity I Bromide (RR-Isomer) (Glycopyrrolate USP Related Compound I (RR-Isomer))

M.F.
M.W. 352.88 79.90
CAT# AR-G02692
CAS# NA
Glycopyrronium Bromide EP Impurity I Bromide (RS-Isomer) (Glycopyrrolate USP Related Compound I (RS-Isomer))

M.F.
M.W. 352.88 79.90
CAT# AR-G02693
CAS# NA
Glycopyrronium Bromide EP Impurity I Bromide (SR-Isomer) (Glycopyrrolate USP Related Compound I (SR-Isomer))

M.F.
M.W. 352.88 79.90
CAT# AR-G02694
CAS# NA
Glycopyrronium Bromide EP Impurity I Bromide (SS-Isomer) (Glycopyrrolate USP Related Compound I (SS-Isomer))

M.F.
M.W. 352.88 79.90
CAT# AR-G02695
CAS# NA
Dehydro Glycopyrrolate Bromide (Mixture of Diastereomers)

M.F.
M.W. 316.42 79.90
CAT# AR-G02701
CAS# NA
Glycopyrronium Bromide EP Impurity I Bromide (Glycopyrrolate USP Related Compound I)

M.F.
M.W. 352.88 79.90
CAT# AR-G02708
CAS# NA