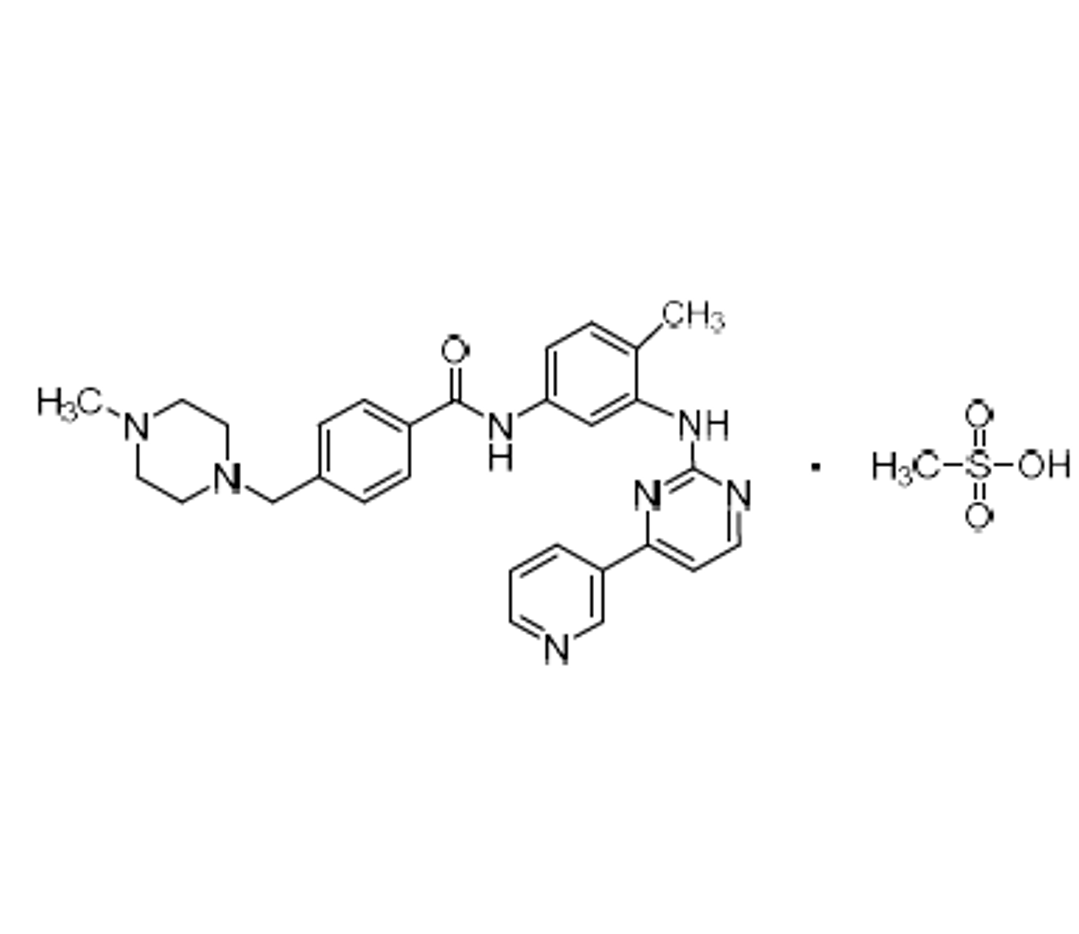
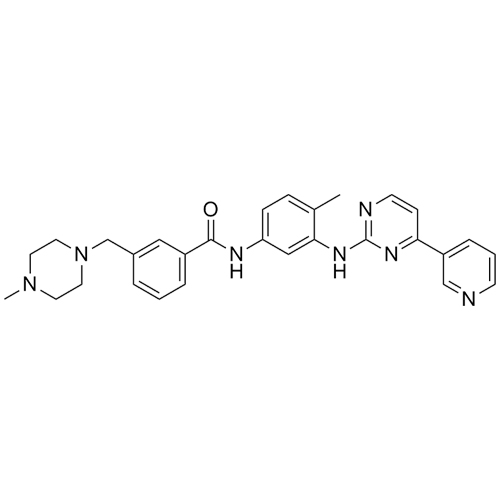
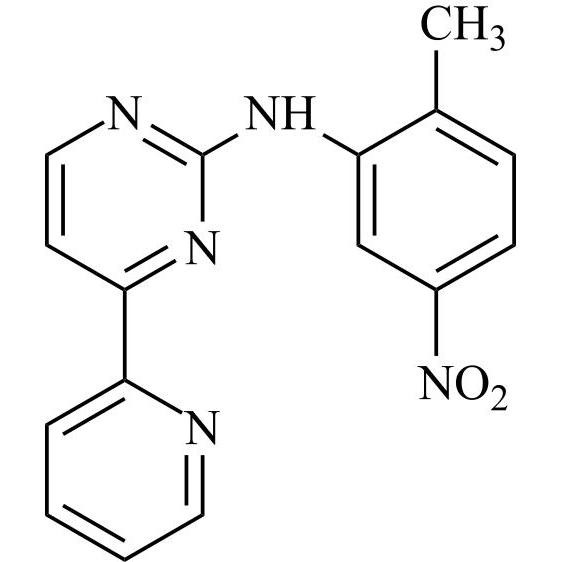
N-(4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)formamide
Imatinib Impurity A is a fully characterized chemical compound used as a reference standard of API Imatinib. The standard offered is compliant with regulatory guidelines. Imatinib Impurity A is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS -
Related products
4-(4-Methylpiperazinomethyl)benzoic Acid, Dihydrochloride

M.F.
M.W. 234.29 72.92
CAT# AR-B00974
CAS# 106261-49-8
N-Nitroso Imatinib EP Impurity C (N-Nitroso Piperazine)

M.F.
M.W. 508.59
CAT# AR-I03054
CAS# 2816369-80-7