- Synonyms2-(1-(3-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)aceticacid;1-(3-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic Acid;[1-(3-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetic Acid; Indomethacin Impurity E;4-Dechloro-3-chloro indomethacin
- Description
2-(1-(3-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)aceticacid;1-(3-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetic Acid;[1-(3-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetic Acid; Indomethacin Impurity E;4-Dechloro-3-chloro indomethacin
Indomethacin EP Impurity E is a fully characterized chemical compound used as a reference standard of API Indomethacin. The standard offered is compliant with regulatory guidelines. Indomethacin EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 807614-94-4
Related products
4-chloro-1-(4-Methoxyphenyl)benzohydrazide sulfonic acid sodium salt

M.F.
M.W. 355.78 22.99
CAT# AR-I01523
CAS# NA
4-chloro-N-(4-methoxyphenyl)benzohydrazide

M.F.
M.W. 276.72
CAT# AR-I01517
CAS# 16390-07-01 (Free Base)
Indomethacin Impurity 26 (Mixture of Diastereomers)
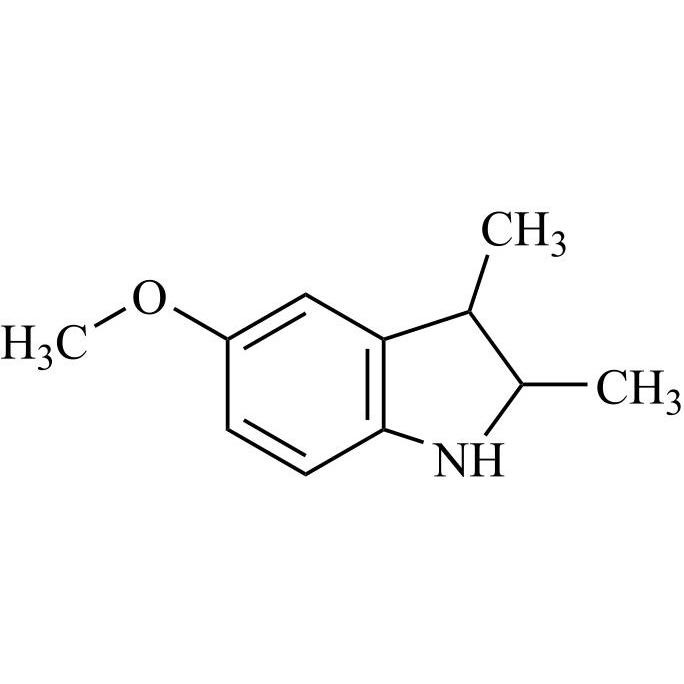
M.F.
M.W. 177.25
CAT# AR-I03677
CAS# 1479098-61-7
O-Desmethyl-N-deschlorobenzoyl Indomethacin-d3

M.F.
M.W. 208.23
CAT# AR-I01532
CAS# 50995-53-4 (non-labelled)
Indomethacin (Indometacin) EP Impurity H-d4 (Indomethacin Methyl Ester-d4)

M.F.
M.W. 375.84
CAT# AR-I03674
CAS# 1217064-61-3