- Synonyms4-ethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl[1,4'-bipiperidine]-1'-carboxylate; (4S)-4-Ethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl Ester [1,4'-Bipiperidine]-1'-carboxylic Acid; 1H-Pyrano...
- Description
4-ethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl[1,4'-bipiperidine]-1'-carboxylate; (4S)-4-Ethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl Ester [1,4'-Bipiperidine]-1'-carboxylic Acid; 1H-Pyrano[3',4':6,7]indolizino[1,2-b]quinoline, [1,4'-Bipiperidine]-1'-carboxylic Acid deriv.; 10-[4-(1-Piperidino)-1-piperidinocarbonyloxy]camptothecin
Irinotecan EP Impurity A is a fully characterized chemical compound used as a reference standard of API Irinotecan. The standard offered is compliant with regulatory guidelines. Irinotecan EP Impurity A is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 103816-16-6
Related products
Irinotecan EP Impurity L HCl (R-Irinotecan HCl)
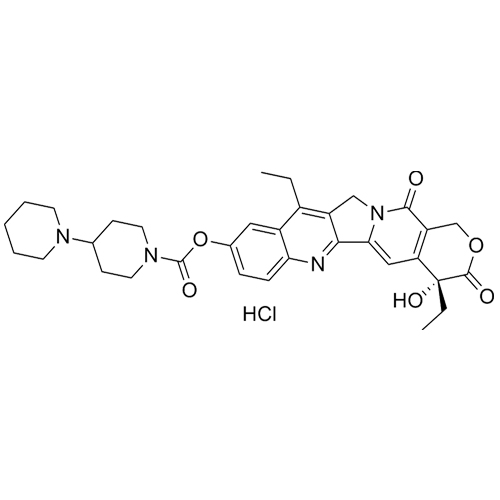
M.F.
M.W. 586.69 36.46
CAT# AR-I01717
CAS# 1992961-26-8
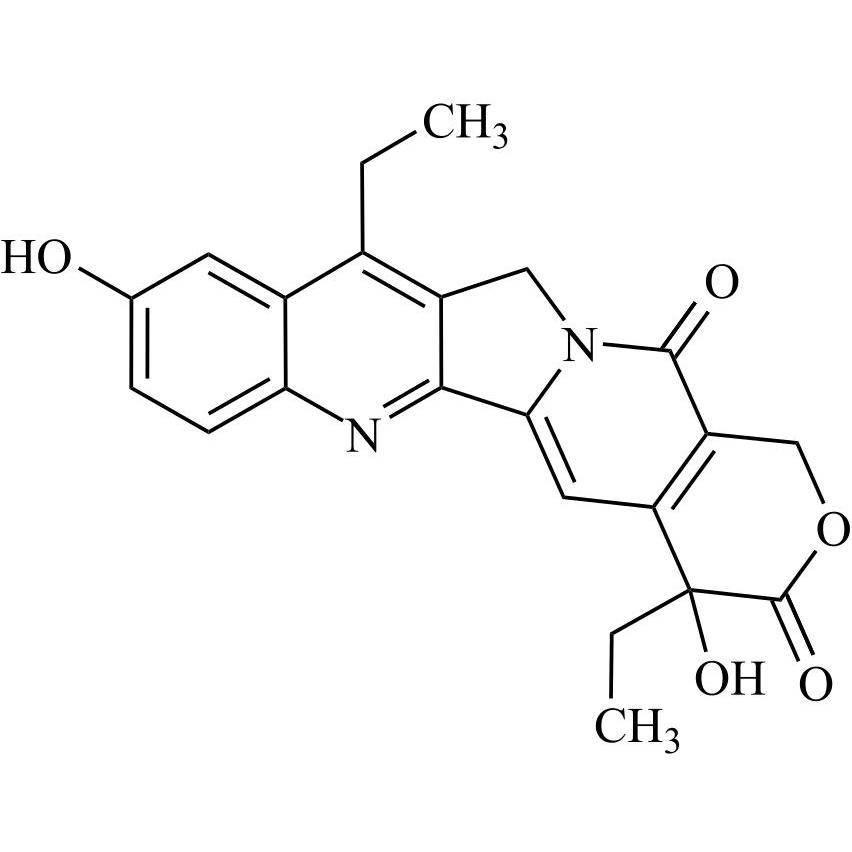
Irinotecan EP Impurity E ((S)-7-Ethyl-10-Hydroxy Camptothecin)

M.F.
M.W. 392.40
CAT# AR-I01722
CAS# 86639-52-3
rac-Irinotecan EP Impurity E (rac-Irinotecan USP Related Compound B, rac-7-Ethyl-10-Hydroxy Camptothecin)
M.F.
M.W. 392.42
CAT# AR-I03936
CAS# NA
N-Nitroso Irinotecan Impurity 24 (N-Nitroso-1,4'-Bipiperidine)
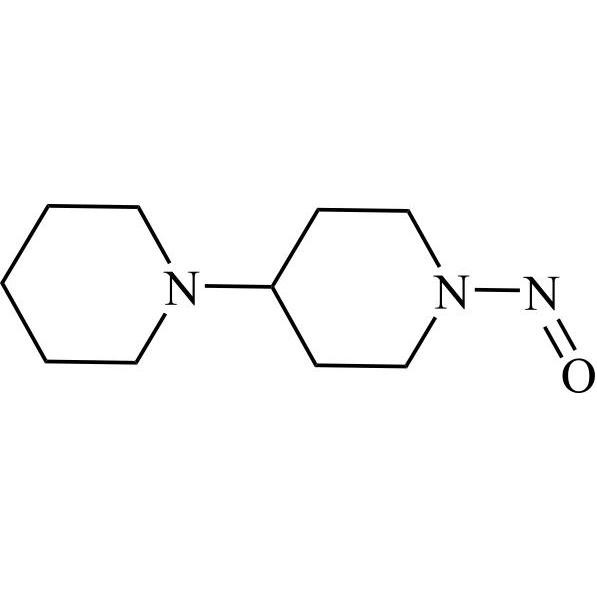
M.F.
M.W. 197.28
CAT# AR-I03944
CAS# 2639422-25-4
Irinotecan EP Impurity E-d3 (Irinotecan USP Related Compound B-d3, (S)-7-Ethyl-10-Hydroxy Camptothecin-d3)
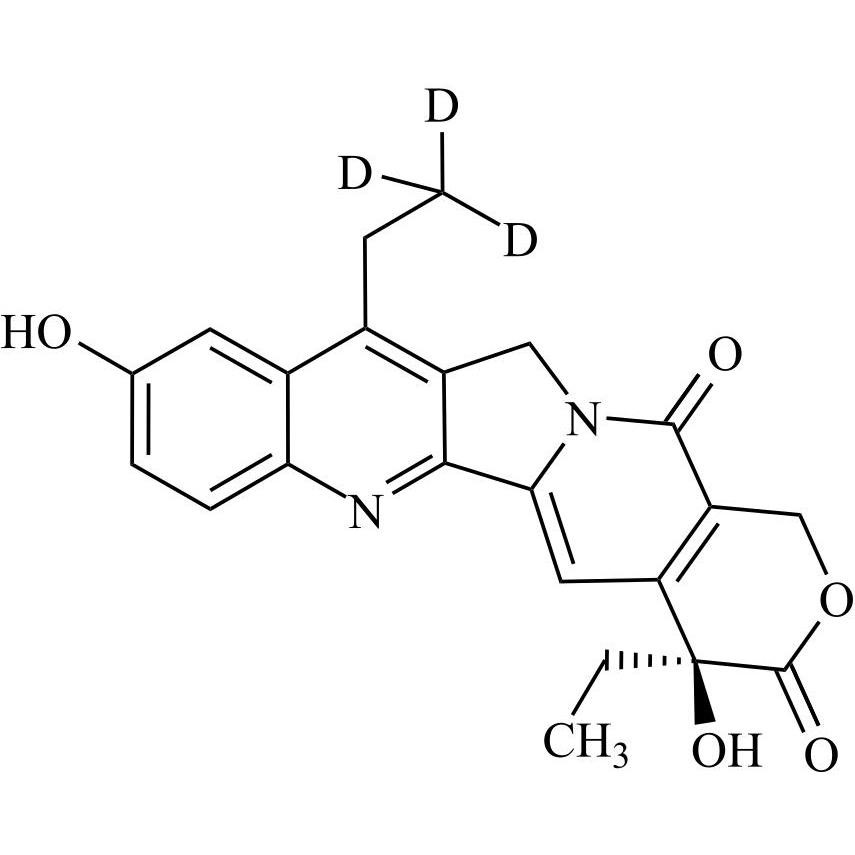
M.F.
M.W. 395.45
CAT# AR-I03935
CAS# NA