Related products
Isavuconazole Impurity 20 Chloride Formate Salt(Mixture of Diastereomers)

M.F.
M.W. 646.69 35.45 46.05
CAT# AR-I03025
CAS# NA
Isavuconazole Impurity 12 Chloride DiHCl (Mixture of Diastereomers)

M.F.
M.W. 761.83 35.45 2*36.46
CAT# AR-I03977
CAS# NA
Isavuconazole Impurity 59 Chloride DiHCl (Mixture of Diastereomers)
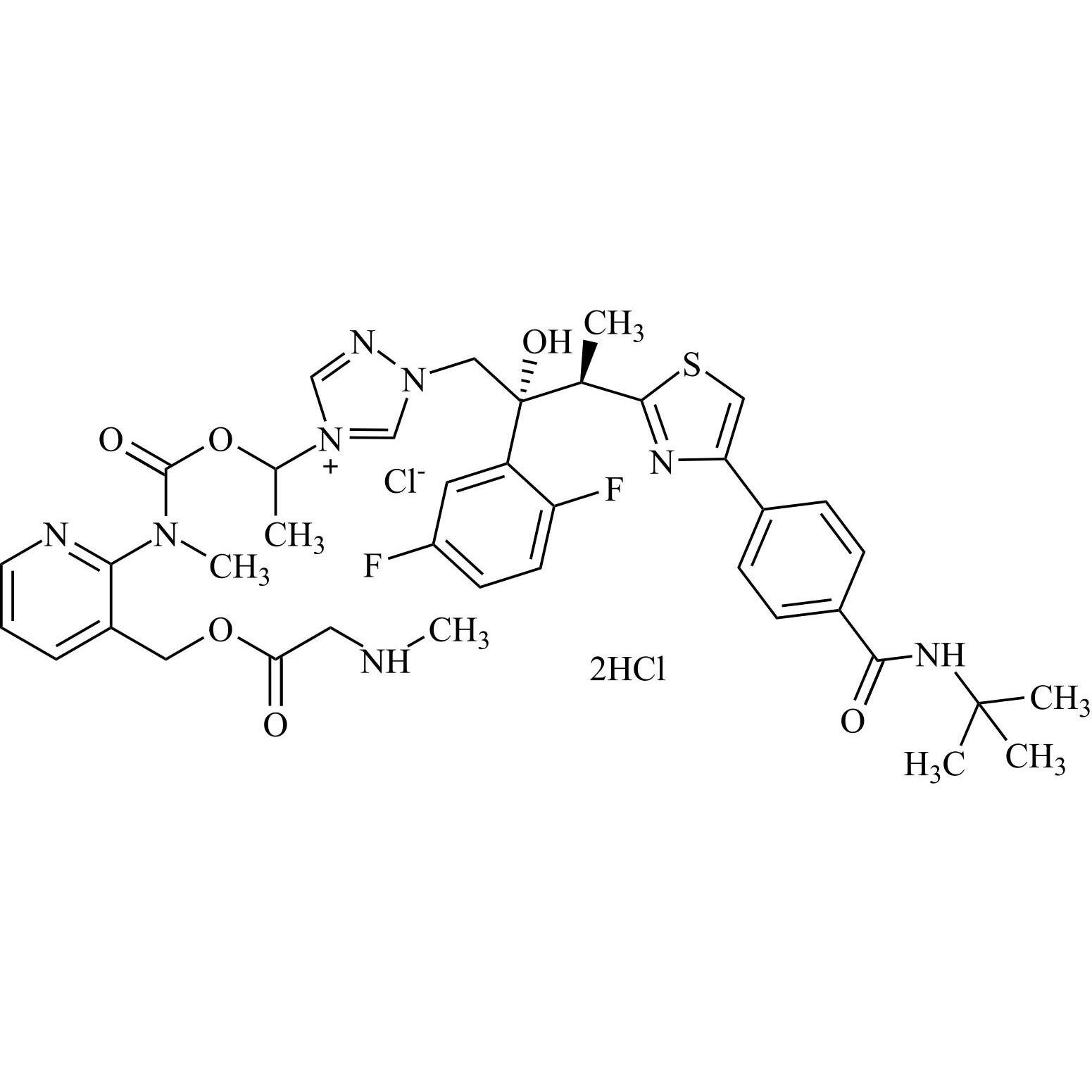
M.F.
M.W. 791.90 35.45 2*36.46
CAT# AR-I04011
CAS# NA
Isavuconazole Impurity 3 Chloride HCl ((2S,3R) Mixture of Diastereomers)

M.F.
M.W. 717.77 35.45 ;36.46
CAT# AR-I04033
CAS# NA
Isavuconazole Impurity 5 Iodide HCl(Mixture of Diastereomers)

M.F.
M.W. 717.8 : 126.9 : 36.5
CAT# AR-I04034
CAS# 2169911-48-0
Isavuconazole Impurity 7 Chloride DiHCl ((2S,3S) Mixture of Diastereomers)
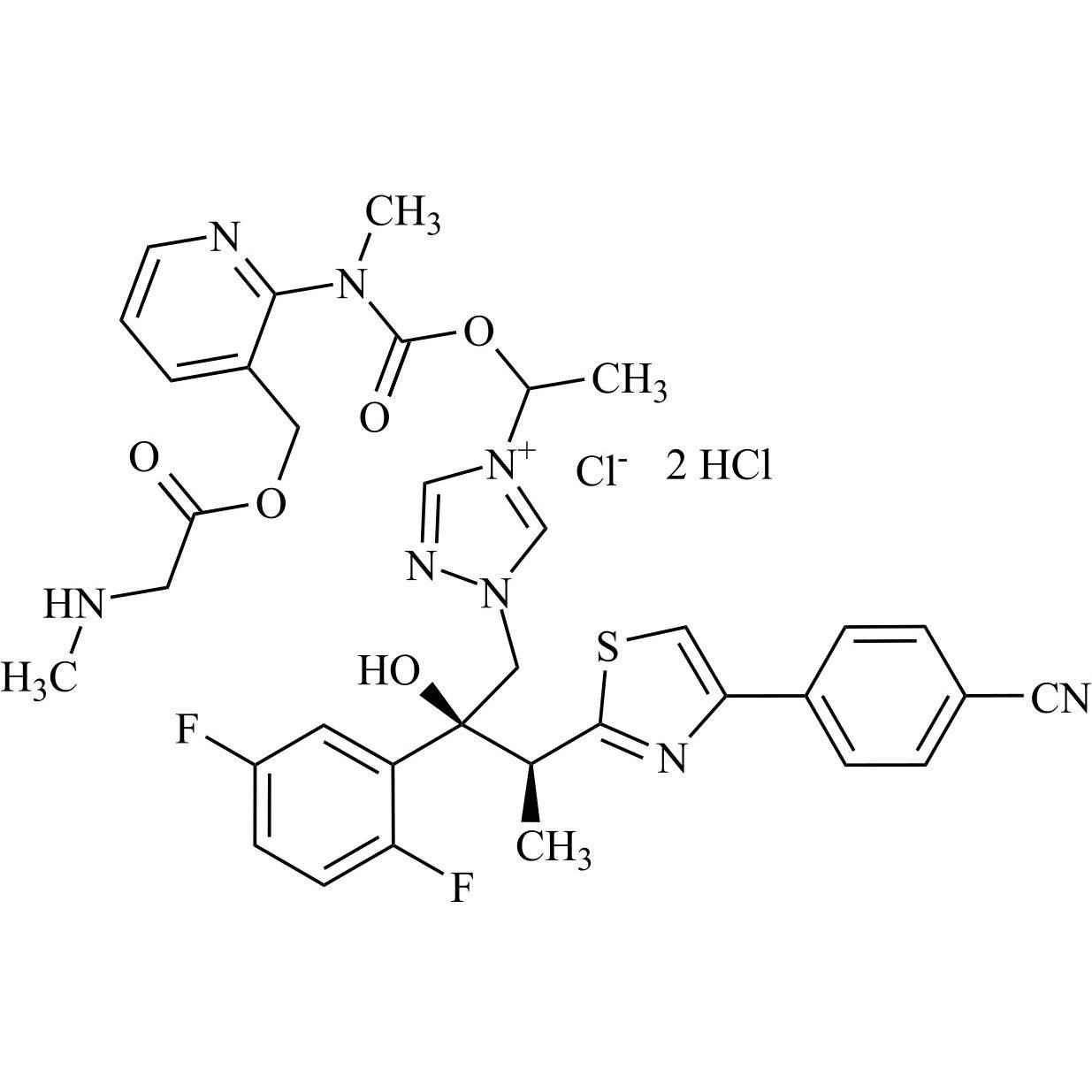
M.F.
M.W. 717.77 35.45 2*36.46
CAT# AR-I04035
CAS# NA
Isavuconazole Impurity 11 Chloride DiHCl (Mixture of Diastereomers)

M.F.
M.W. 645.71 35.45 2*36.46
CAT# AR-I04036
CAS# NA
Isavuconazole Impurity 29 Chloride (Mixture of Diastereomers)

M.F.
M.W. 817.90 35.45
CAT# AR-I04037
CAS# NA
Isavuconazole Imino Impurity Cl HCl Salt (2R,3R)

M.F.
M.W. 791.9 : 35.5 : 2(36.5)
CAT# AR-I04347
CAS# NA
Isavuconazole Impurity 7 Iodide HCl ((2S,3S) Mixture of Diastereomers)

M.F.
M.W. 717.8 : 126.9 : 36.5
CAT# AR-I04349
CAS# NA