Related products
Labetalol EP Impurity A HCl (Mixture of Diastereomers)
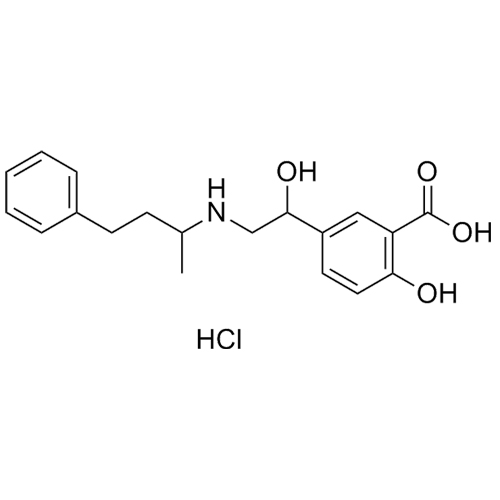
M.F.
M.W. 329.40 36.46
CAT# AR-L01004
CAS# 1391051-99-2 (free base)
Labetalol EP Impurity B HCl (Mixture of Diastereomers)
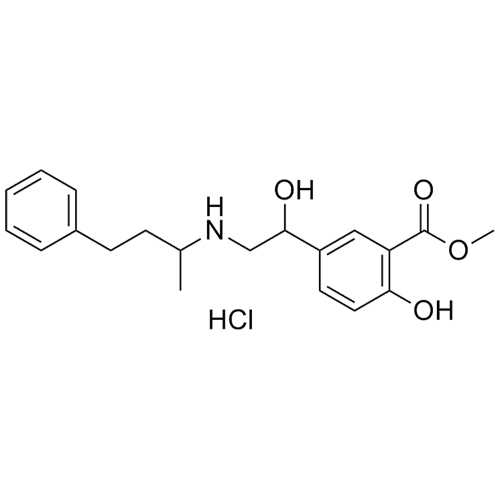
M.F.
M.W. 343.43 36.46
CAT# AR-L01005
CAS# 33778-93-7
5-(2-((4-cyclohexylbutan-2-yl)amino)-1-hydroxyethyl)-2-hydroxybenzamide
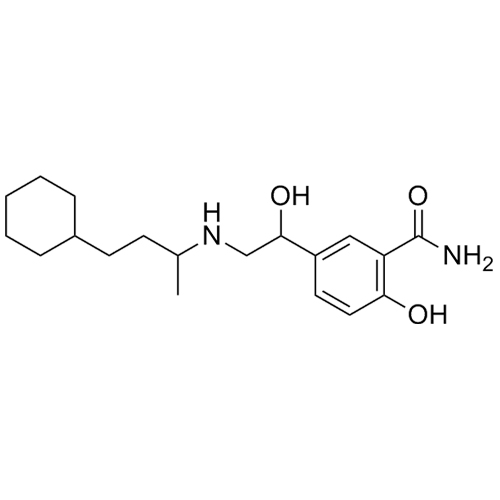
M.F.
M.W. 334.46
CAT# AR-L01007
CAS# NA
Labetalol EP Impurity A HCl ((R,R)-isomer and enantiomer)
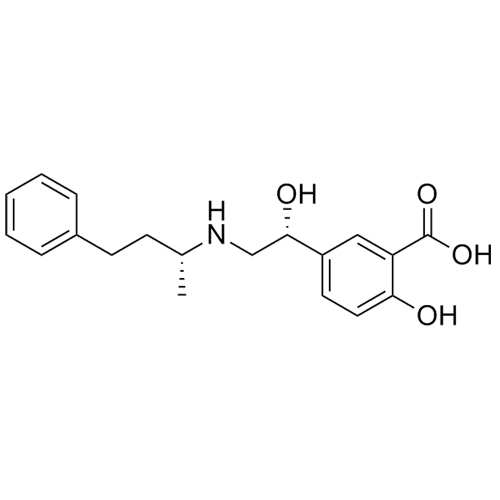
M.F.
M.W. 329.40 36.46
CAT# AR-L01008
CAS# NA
Labetalol EP Impurity A HCl ((R,S)-isomer and enantiomer)
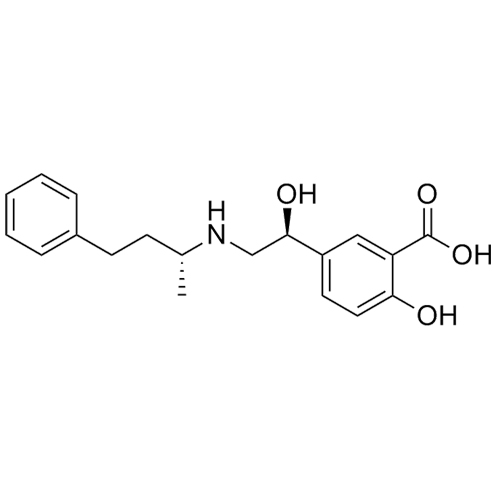
M.F.
M.W. 329.40 36.46
CAT# AR-L01009
CAS# NA
Labetalol EP Impurity G HCl (Mixture of Diastereomers)
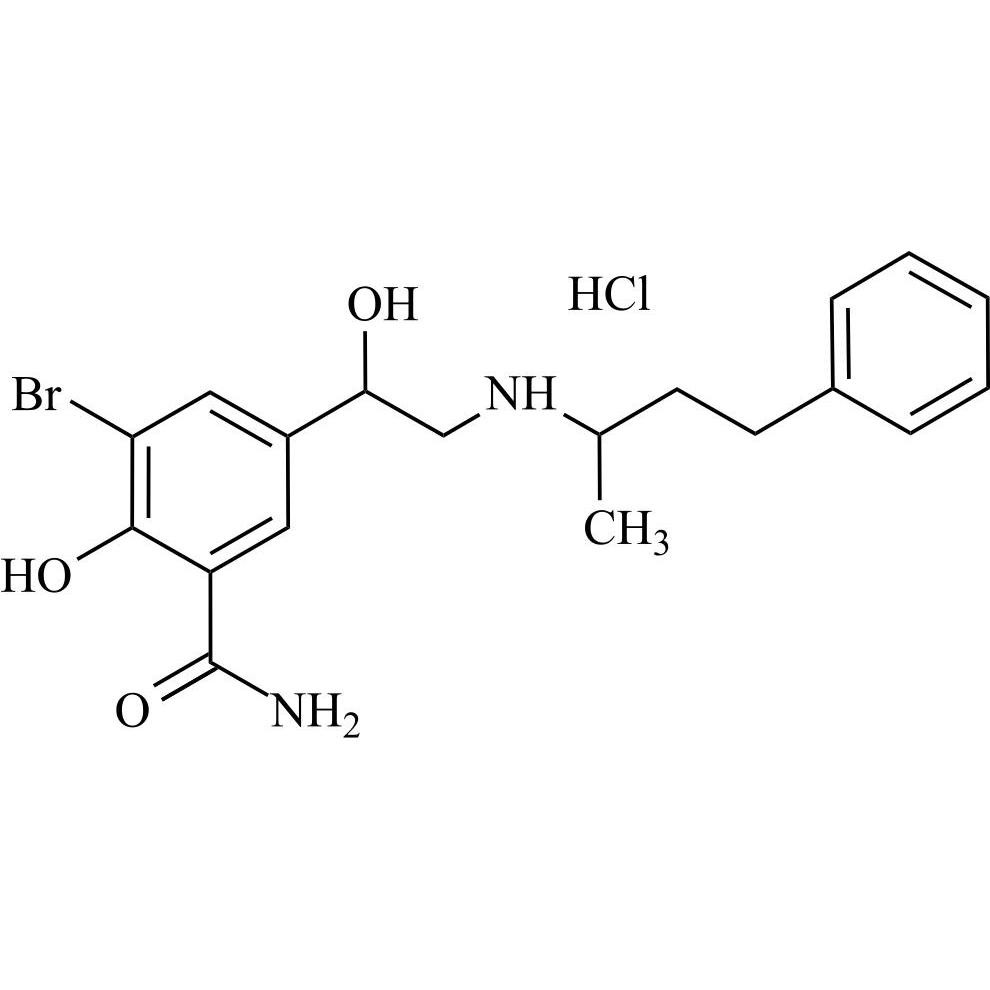
M.F.
M.W. 407.31 36.46
CAT# AR-L06360
CAS# NA
Labetalol EP Impurity A HCl ((R,R)-isomer and enantiomer)
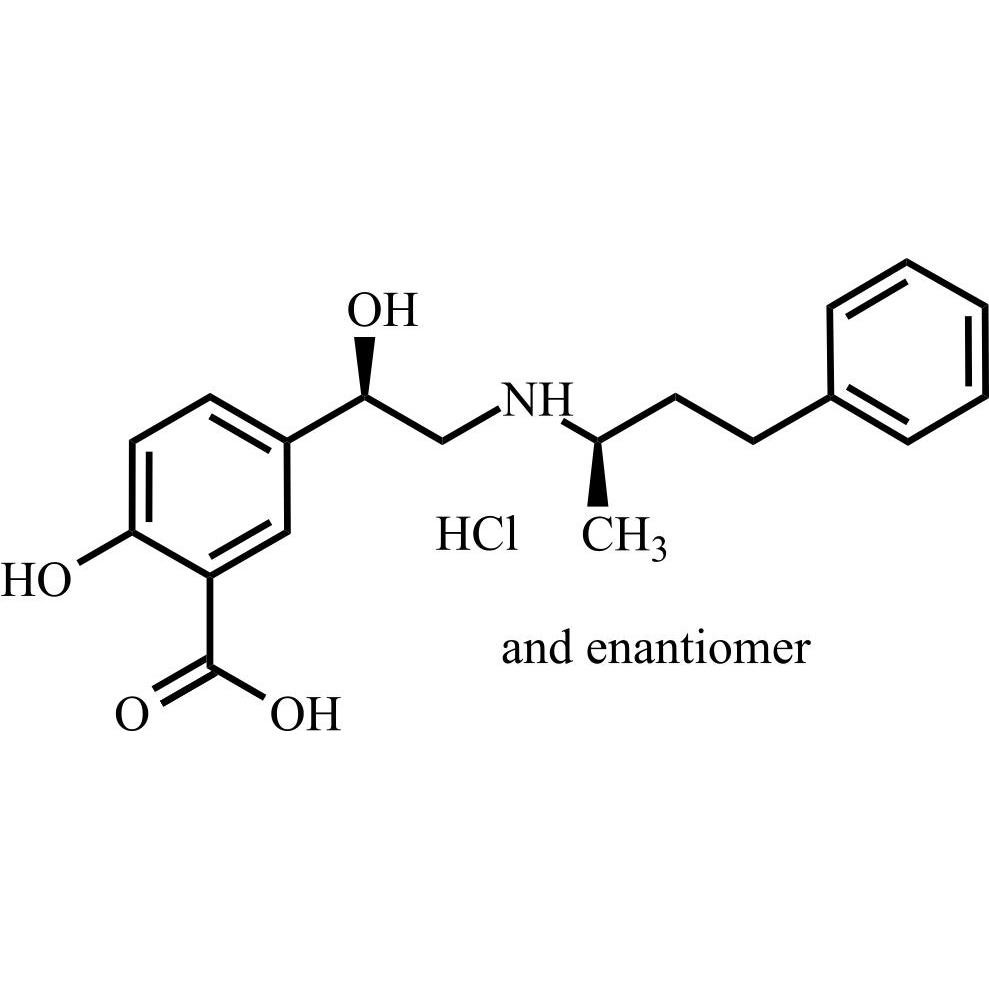
M.F.
M.W. 329.40 36.46
CAT# AR-L06369
CAS# NA
Labetalol EP Impurity A HCl ((R,S)-isomer and enantiomer)
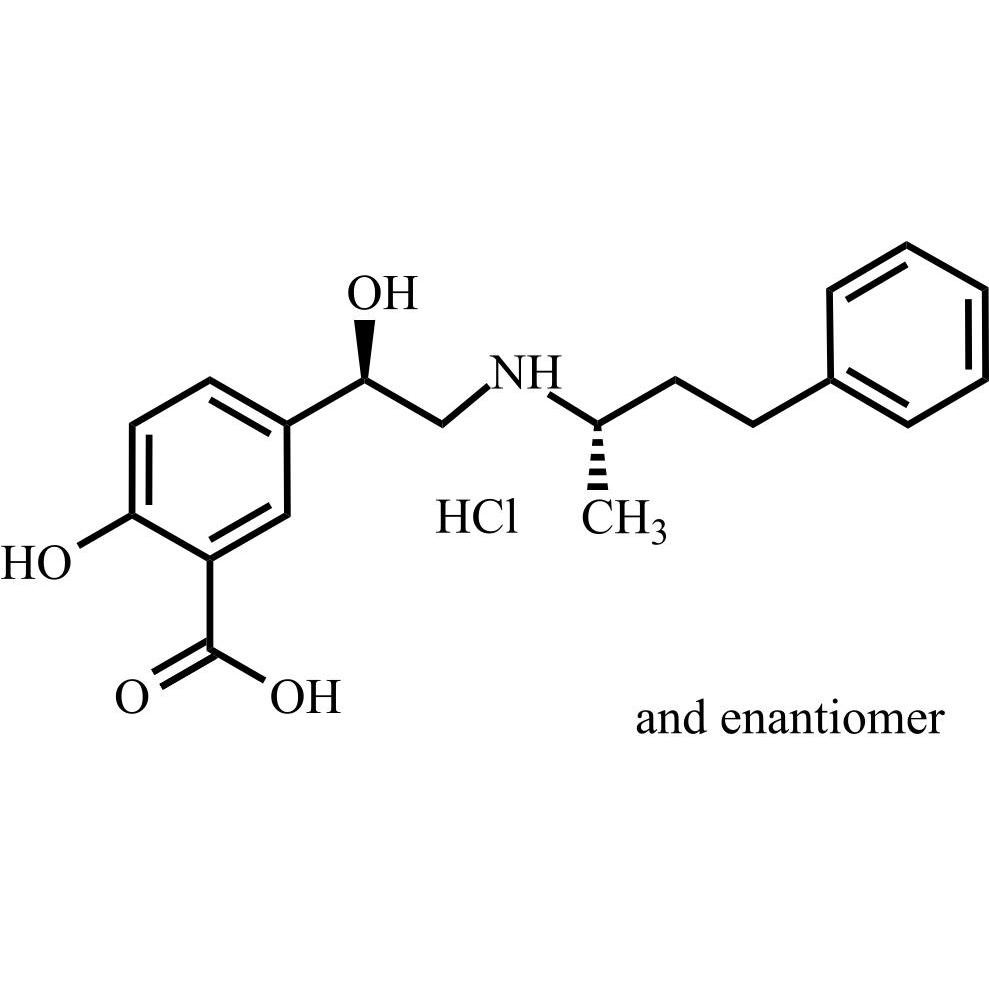
M.F.
M.W. 329.40 36.46
CAT# AR-L06370
CAS# NA
N-Nitroso Labetalol EP Impurity A (N-Nitroso Labetalol USP Related Compound A) (Mixture of Diastereomers)
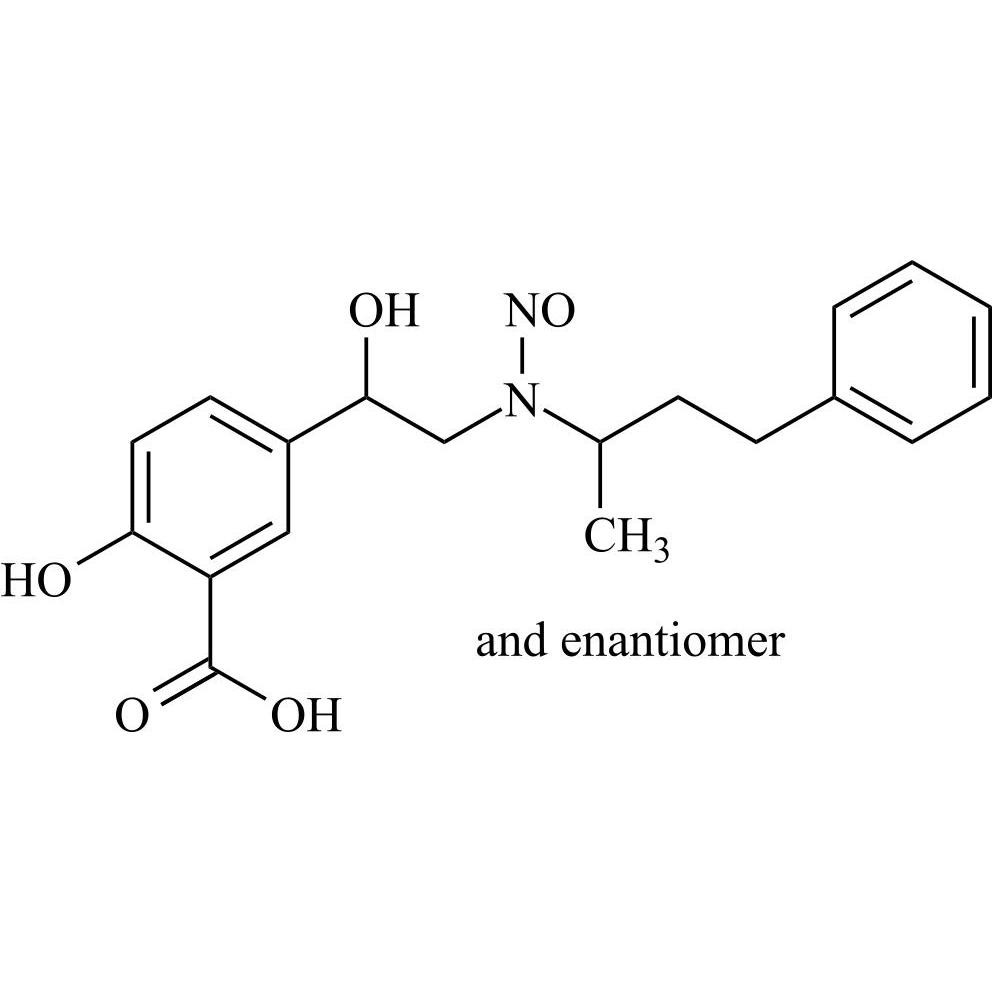
M.F.
M.W. 358.39
CAT# AR-L06359
CAS# NA
N-Nitroso Labetalol EP Impurity F (N-Nitroso Labetalol USP Related Compound F (Free Form))
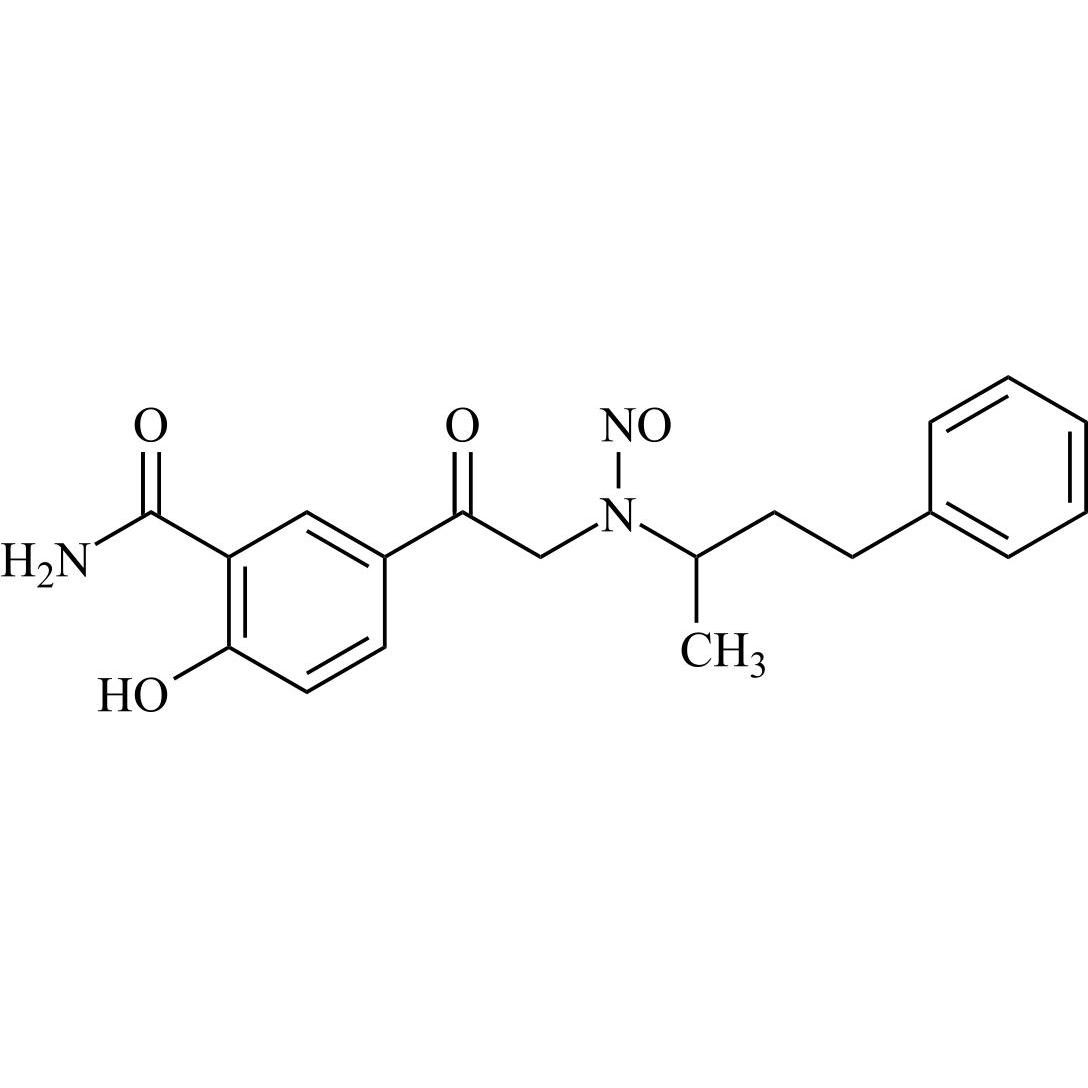
M.F.
M.W. 355.39
CAT# AR-L06363
CAS# NA
N-Nitroso Labetalol EP Impurity A (N-Nitroso Labetalol USP Related Compound A) ((R,R)-Isomer and Enantiomer)
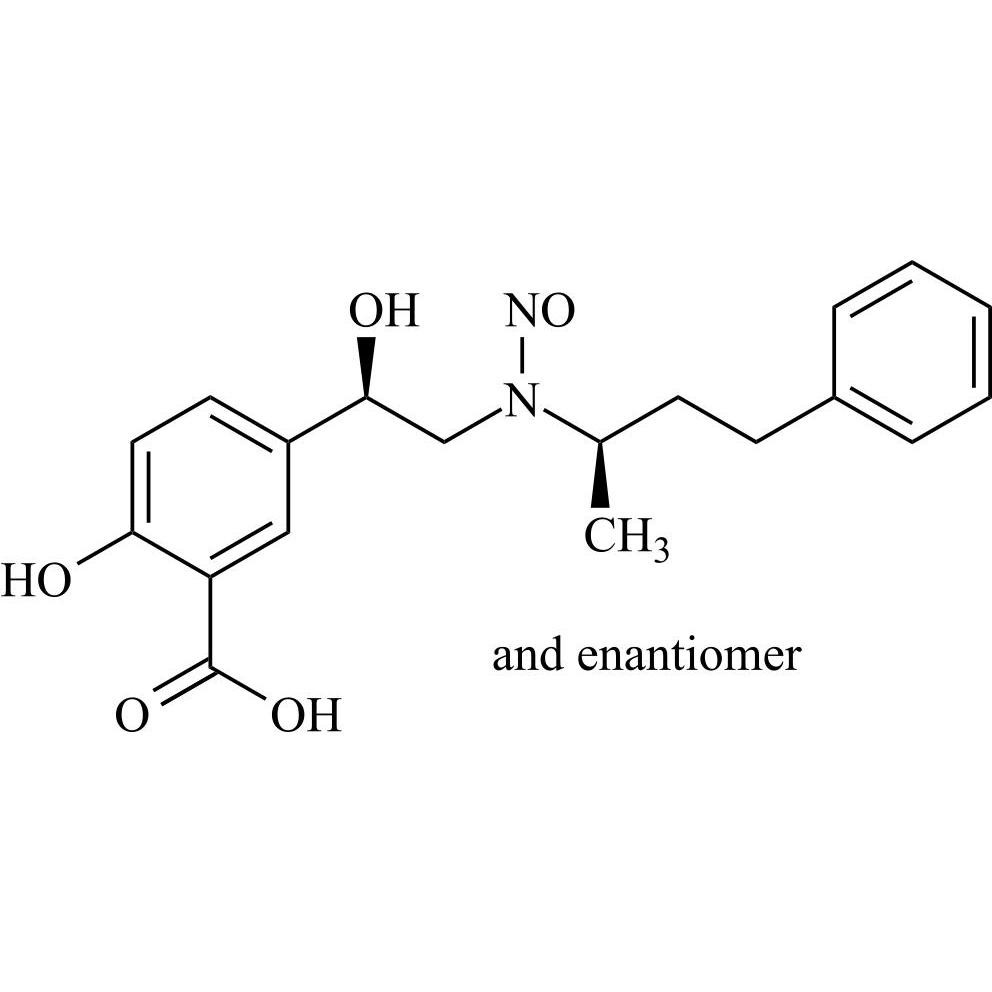
M.F.
M.W. 358.39
CAT# AR-L06366
CAS# NA
N-Nitroso Labetalol EP Impurity A (N-Nitroso Labetalol USP Related Compound A) ((R,S)-Isomer and Enantiomer)
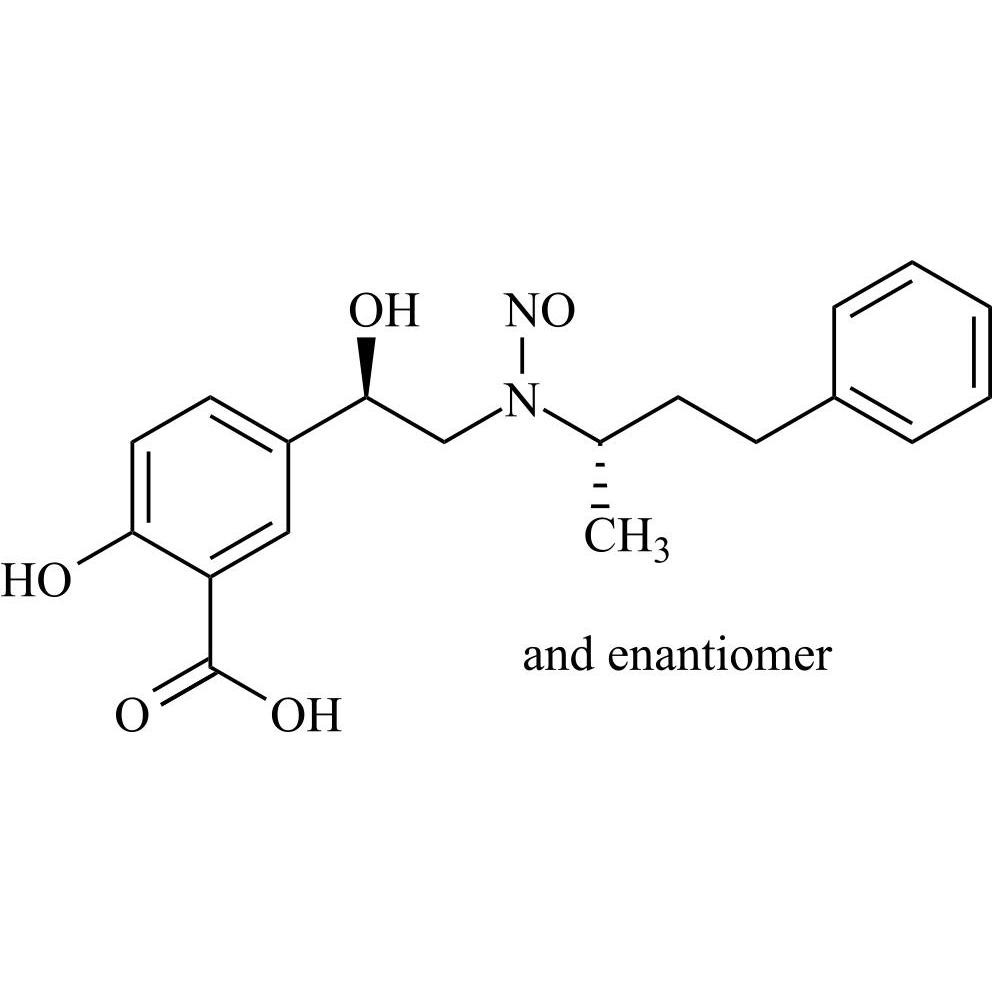
M.F.
M.W. 358.39
CAT# AR-L06367
CAS# NA
Labetalol-d5 HCl (Mixture of Diastereomers)
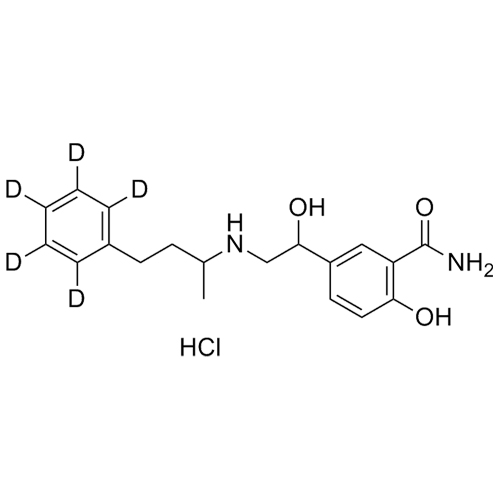
M.F.
M.W. 333.44 36.46
CAT# AR-L01002
CAS# 32780-64-6 (non-labelled)












![Show details for 5[(1,3-Diphenylpropan-2-yl)glycyl]-2-hydroxybenzamide Picture of 5[(1,3-Diphenylpropan-2-yl)glycyl]-2-hydroxybenzamide](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-L06072.jpg?size=256)

















