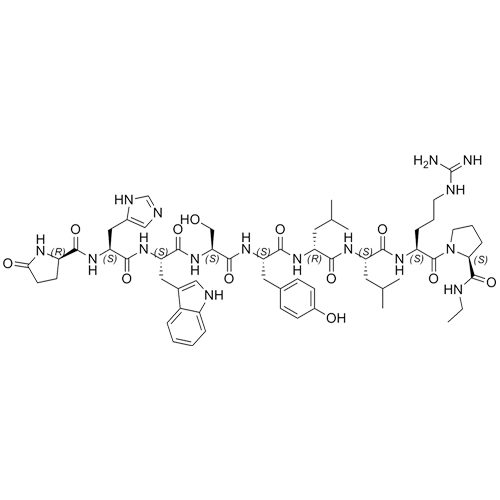
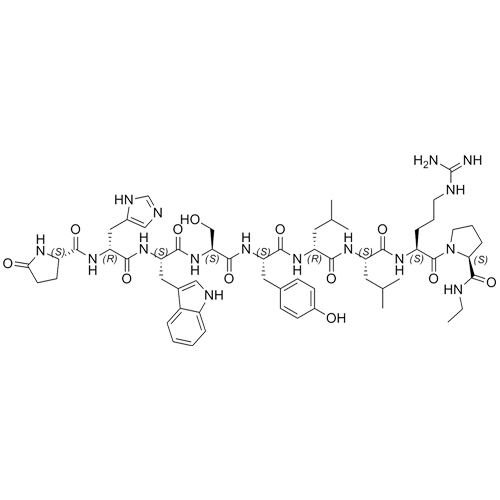
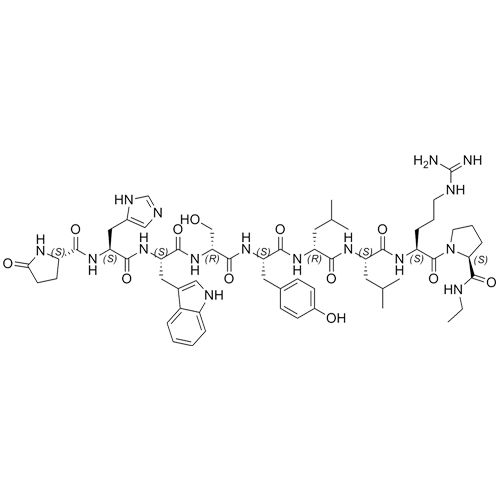
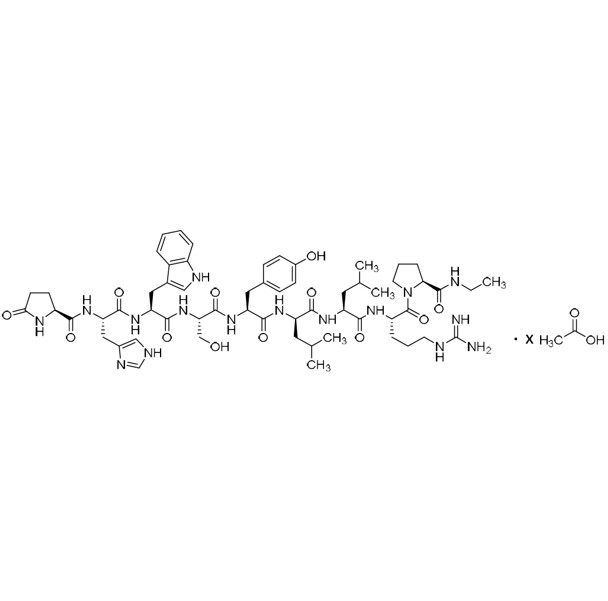
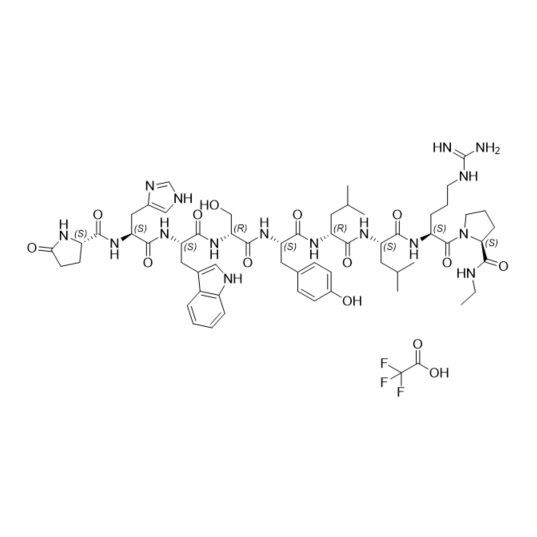
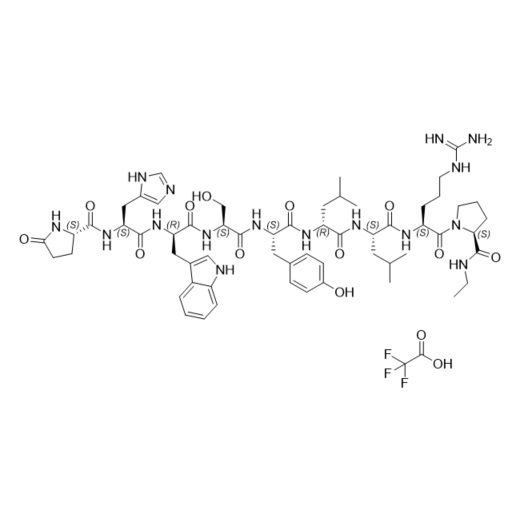
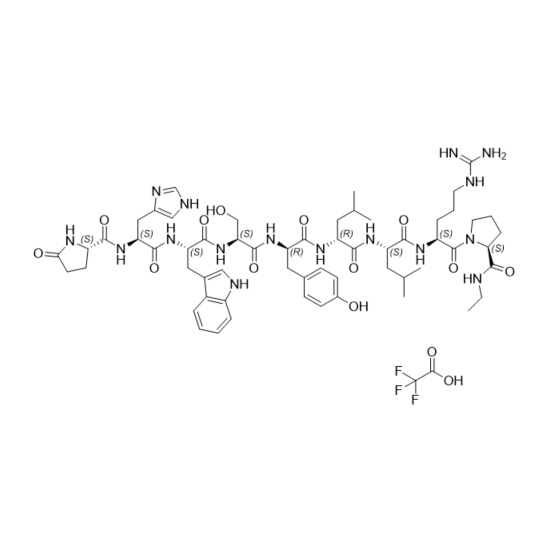
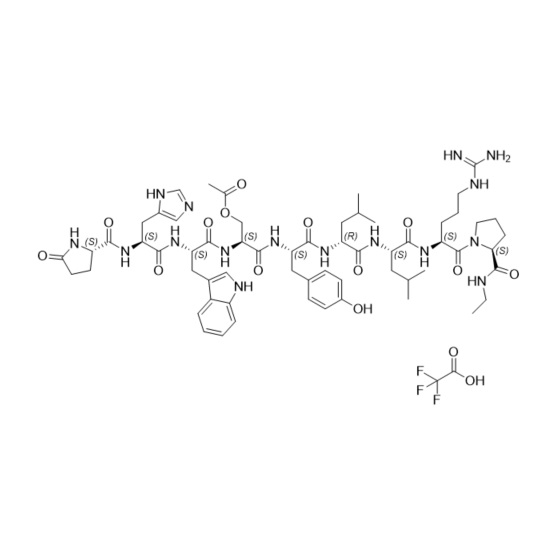
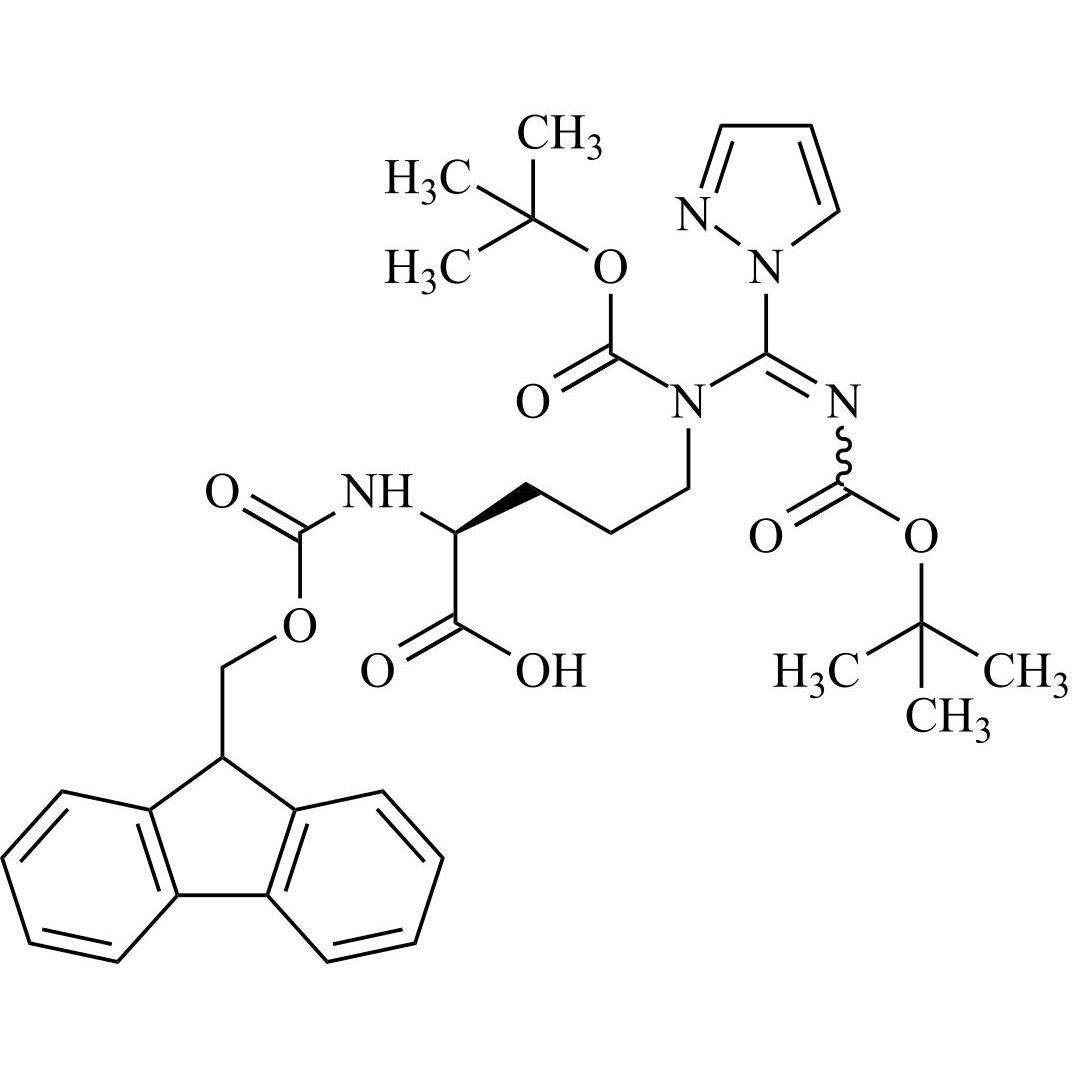
- Synonyms(S)-1-((3S,6S,9S,12S,15R,18R,21S)-3-((1H-imidazol-5-yl)methyl)-6-((1H-indol-3-yl)methyl)-21-(3-guanidinopropyl)-12-(4-hydroxybenzyl)-9-(hydroxymethyl)-15,18-diisobutyl-1,4,7,10,13,16,19-heptaoxo-1-((S)-5-oxopyrrolidin-2-yl)-2,5,8,11,14,17,20-heptaazadocosan-22-oyl)-N-ethylpyrrolidine-2-carboxamide
- Description
Related products
Leuprorelin EP Impurity G (Leuprolide EP Impurity G)
M.F.
M.W. 1209.42
CAT# AR-L01442
CAS# 112710-57-3
Leuprorelin (Leuprolide) Impurity 7 Ditrifluoroacetate

M.F.
M.W. 747.90 2*114.02
CAT# AR-L06936
CAS# NA
Leuprorelin (Leuprolide) Impurity 8 Ditrifluoroacetate

M.F.
M.W. 774.97 2*114.02
CAT# AR-L06937
CAS# NA
Leuprorelin (Leuprolide) EP Impurity I Ditrifluoroacetate

M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06920
CAS# NA
Leuprorelin (Leuprolide) Impurity 10 Ditrifluoroacetate

M.F.
M.W. 1085.23 2* 114.02
CAT# AR-L06922
CAS# NA
Leuprorelin (Leuprolide) Impurity 11 Trifluoroacetate

M.F.
M.W. 702.73 114.02
CAT# AR-L06923
CAS# NA
5-9-Leuprorelin Ditrifluoroacetate (5-9-Leuprolide Ditrifluoroacetate)

M.F.
M.W. 687.89 2*114.02
CAT# AR-L06925
CAS# NA
Leuprorelin (Leuprolide) Impurity 2 Tritrifluoroacetate

M.F.
M.W. 1098.33 3*114.02
CAT# AR-L06927
CAS# NA
Leuprorelin (Leuprolide) Impurity 3 Ditrifluoroacetate

M.F.
M.W. 774.97 2*114.02
CAT# AR-L06928
CAS# NA
Leuprorelin (Leuprolide) Impurity 4 ((3-9)-Leuprorelin (Leuprolide)) Ditrifluoroacetate

M.F.
M.W. 961.18 2*114.02
CAT# AR-L06929
CAS# NA
Leuprorelin (Leuprolide) Impurity 5 (Des-Ser4-Leuprorelin (Leuprolide)) Ditrifluoroacetate

M.F.
M.W. 1122.34 2*114.02
CAT# AR-L06930
CAS# NA
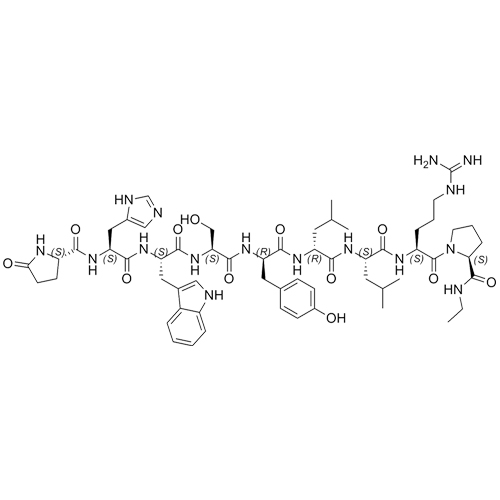
Leuprorelin (Leuprolide) EP Impurity G Ditrifluoroacetate

M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06932
CAS# NA
Leuprorelin (Leuprolide) EP Impurity B Ditrifluoroacetate

M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06933
CAS# NA
Leuprorelin (Leuprolide) EP Impurity C Ditrifluoroacetate

M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06934
CAS# NA
Leuprorelin (Leuprolide) EP Impurity A Ditrifluoroacetate

M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06935
CAS# NA
Leuprorelin (Leuprolide) EP Impurity E Ditrifluoroacetate

M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06939
CAS# NA
Leuprorelin (Leuprolide) EP Impurity H Ditrifluoroacetate

M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06940
CAS# NA
Leuprorelin (Leuprolide) EP Impurity F Ditrifluoroacetate
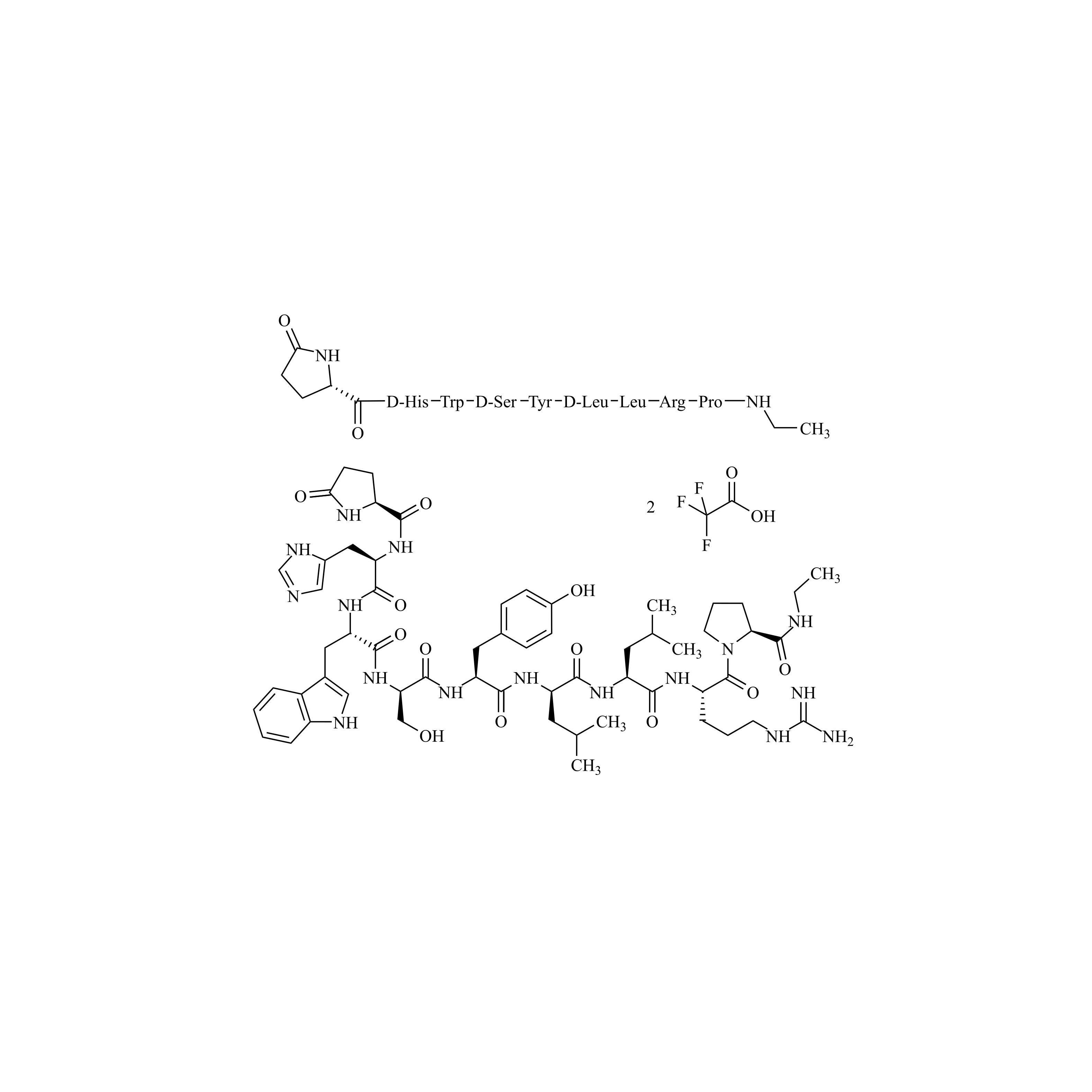
M.F.
M.W. 1209.42 2*114.02
CAT# AR-L06941
CAS# NA
Leuprorelin (Leuprolide) EP Impurity D Ditrifluoroacetate

M.F.
M.W. 1251.46 2*114.02
CAT# AR-L06942
CAS# NA
Leuprorelin (Leuprolide)-d10 Trifluoroacetic Acid Salt
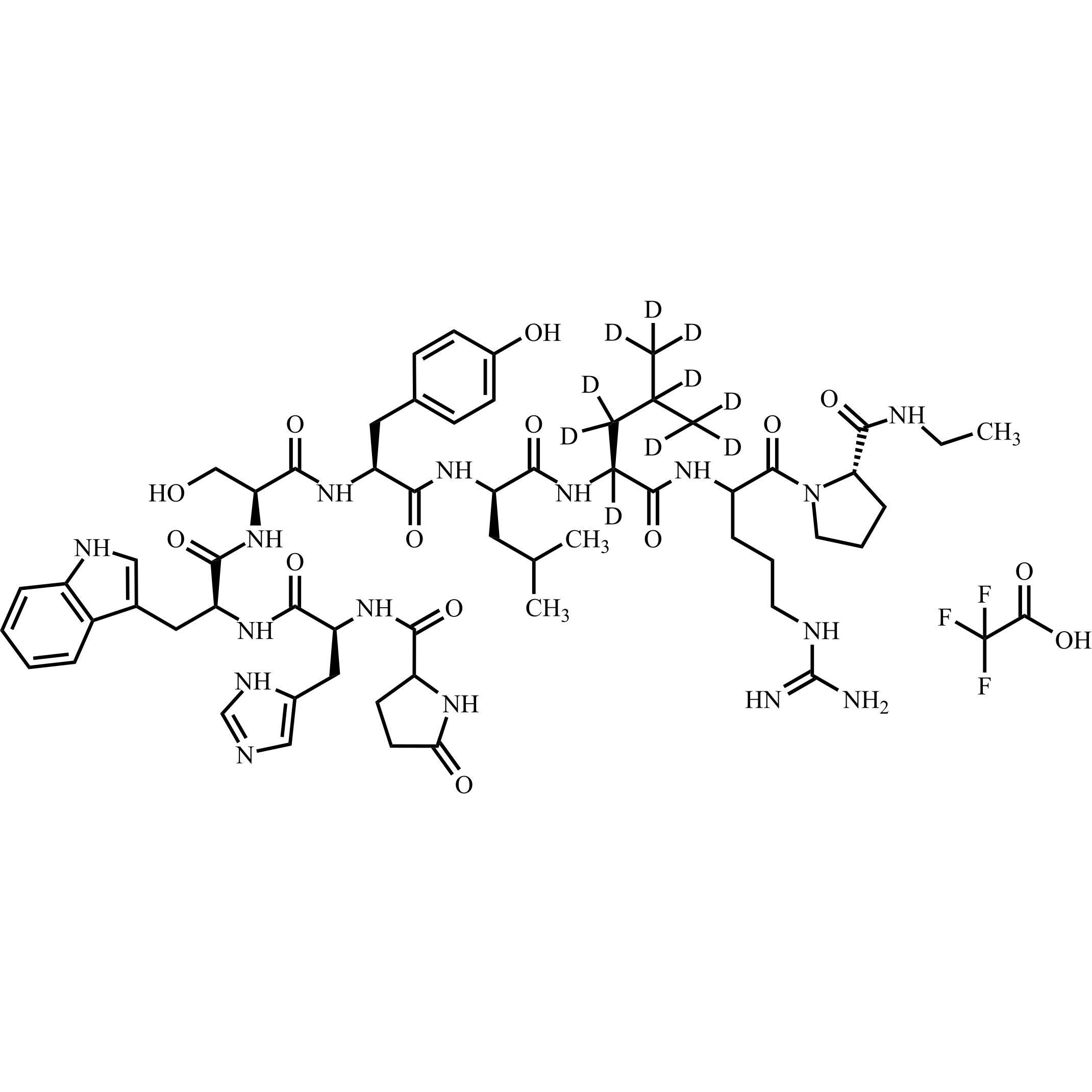
M.F.
M.W. 1219.48 114.02
CAT# AR-L06921
CAS# NA