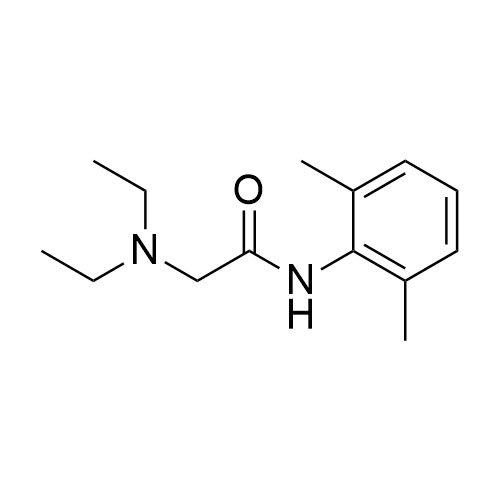
- Synonyms2,2'-azanediylbis(N-(2,6-dimethylphenyl)acetamide);2,2’-Iminobis(N-(2,6-Dimethylphenyl)acetiamide
- Description
2,2'-azanediylbis(N-(2,6-dimethylphenyl)acetamide);2,2’-Iminobis(N-(2,6-Dimethylphenyl)acetiamide
Lidocaine EP Impurity E is a fully characterized chemical compound used as a reference standard of API Lidocaine. The standard offered is compliant with regulatory guidelines. Lidocaine EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 745798-07-6
Related products
N-Desethyl Lidocaine Hydrochloride Monohydrate EP Impurity K

M.F.
M.W. 192.26
CAT# AR-L07197
CAS# 42459-27-8
N-Desethyl Lidocaine Hydrochloride Monohydrate EP Impurity K HCl

M.F.
M.W. 192.26 36.46
CAT# AR-L07217
CAS# 35891-84-0
N-Nitroso Lidocaine EP Impurity H (N-Nitroso Lidocaine USP Related Compound H)
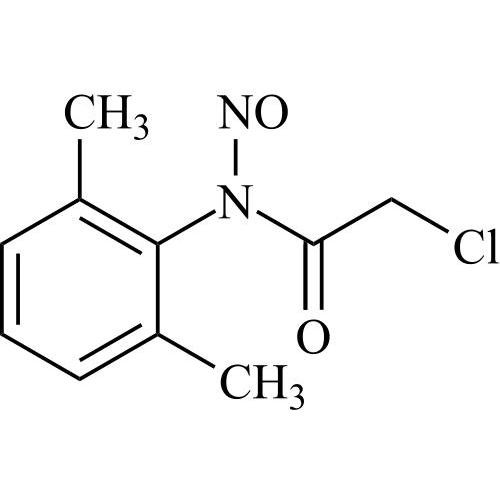
M.F.
M.W. 226.66
CAT# AR-L07192
CAS# NA
N-Nitroso Lidocaine EP Impurity B (N-Nitroso Lidocaine N-Oxide)

M.F.
M.W. 279.34
CAT# AR-L07193
CAS# NA
N-Nitroso-N-Desethyl Lidocaine Hydrochloride Monohydrate EP Impurity K
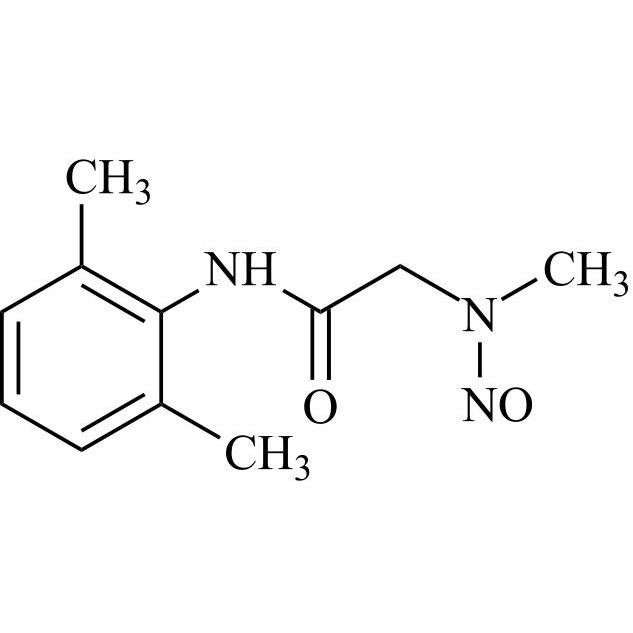
M.F.
M.W. 221.26
CAT# AR-L07215
CAS# NA
Analytical Services - Stability Testing of N-Nitroso Lidocaine

M.F.
M.W. 263.34
CAT# AR-L07188_AS
CAS# NA
Lidocaine EP Impurity A-d6 HCl (Bupivacaine EP Impurity F-d6 HCl, Ropivacaine EP Impurity H-d6 HCl, Ropivacaine USP Related Compound A-d6)

M.F.
M.W. 127.22 36.46
CAT# AR-L07218
CAS# NA