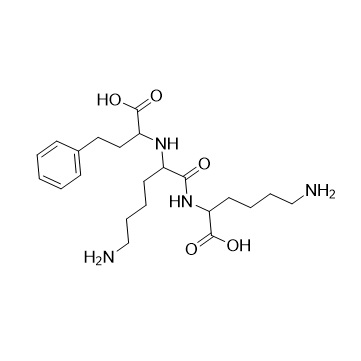
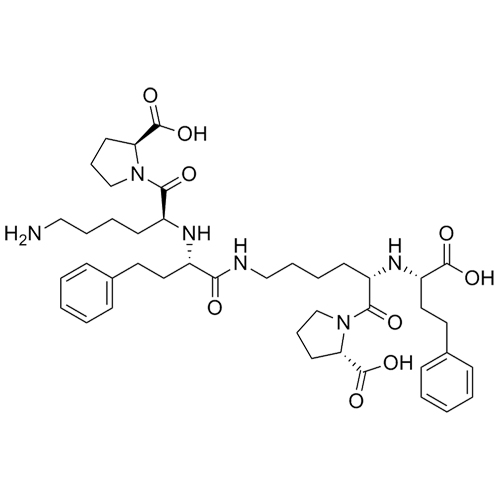
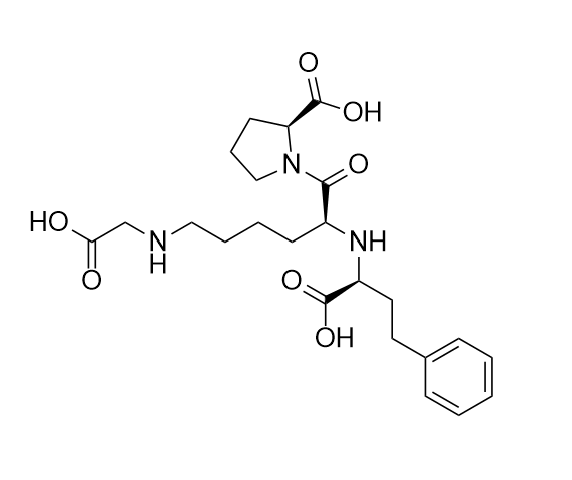
- Synonyms(R)-1-[N2-(1-Carboxy-3-phenylpropyl)-L-lysyl]L-proline Sodium;N2-[(1R)-1-Carboxy-3-phenylpropyl)-L-lysyl]L-proline Sodium;(R)-Lisinopril Sodium Salt
- Description
(R)-1-[N2-(1-Carboxy-3-phenylpropyl)-L-lysyl]L-proline Sodium;N2-[(1R)-1-Carboxy-3-phenylpropyl)-L-lysyl]L-proline Sodium;(R)-Lisinopril Sodium Salt
Lisinopril EP Impurity E is a fully characterized chemical compound used as a reference standard of API Lisinopril. The standard offered is compliant with regulatory guidelines. Lisinopril EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 85955-59-5(free acid)
Related products
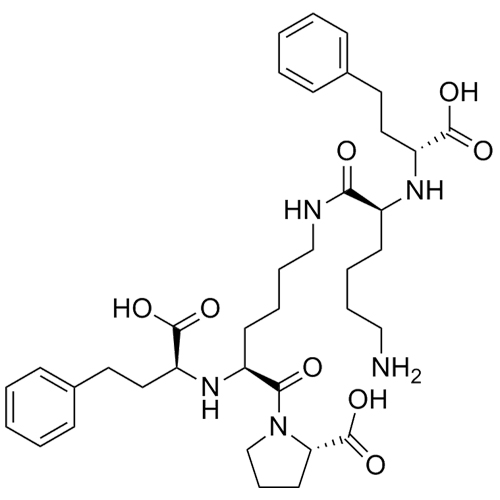
Lisinopril Impurity H (Dimer Impurity, Mixture of Diastereomers)
M.F.
M.W. 695.86
CAT# AR-L01752
CAS# NA
N-(1-Carboxy-3-phenylpropyl)-S-lisinopril Trisodium Salt (Mixture of diastereomers)
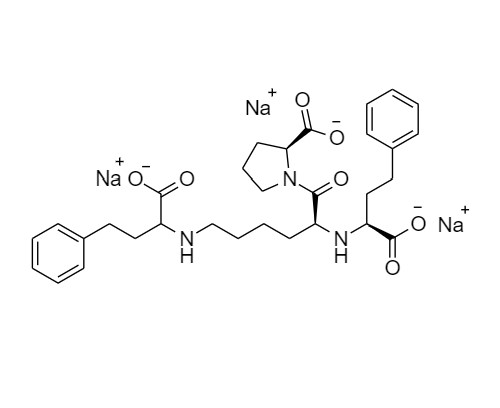
M.F.
M.W. 633.67
CAT# AR-L06104
CAS# NA
Lisinopril Impurity 12 (2,2,2-trifluoroethyl formate)

M.F.
M.W. 128.05
CAT# AR-L07527
CAS# 32042-38-9
Lisinopril EP Impurity C HCl ((S,S,S)-Diketopiperazine HCl)

M.F.
M.W. 387.48 36.46
CAT# AR-L07534
CAS# 328385-86-0 (free base)
Lisinopril EP Impurity D HCl ((R,S,S)-Diketopiperazine HCl)
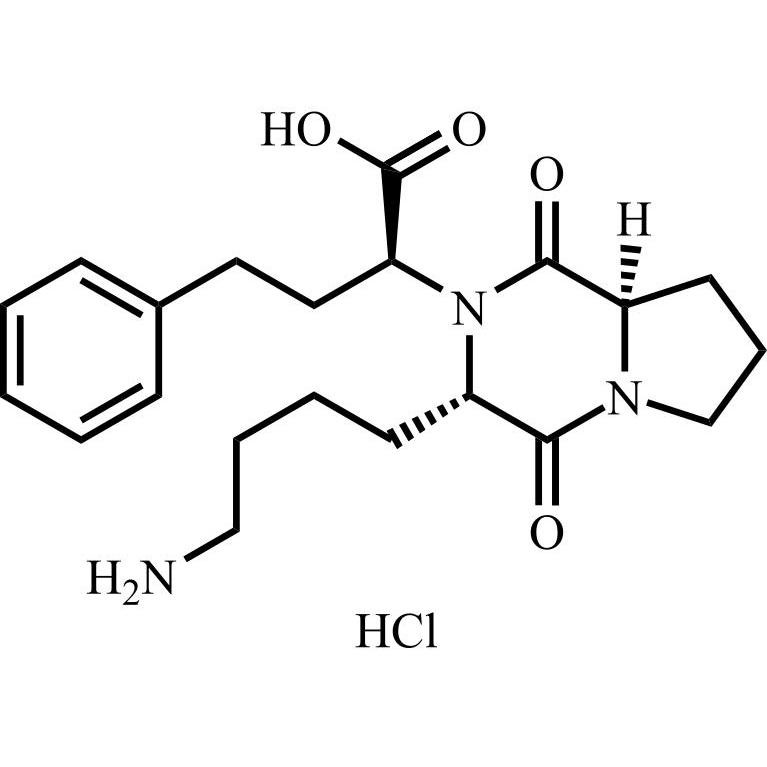
M.F.
M.W. 387.48 36.46
CAT# AR-L07548
CAS# NA