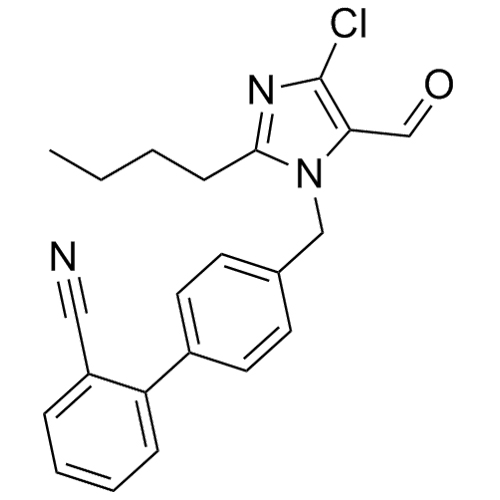
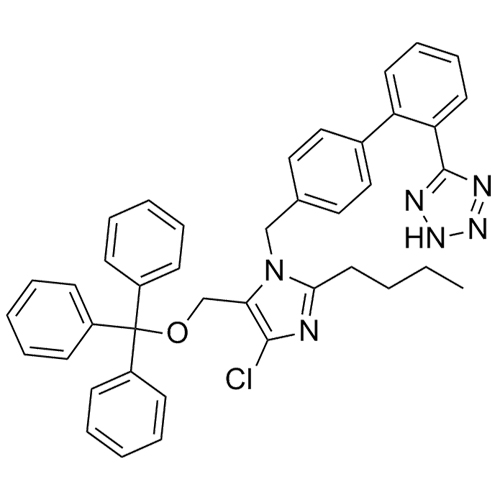
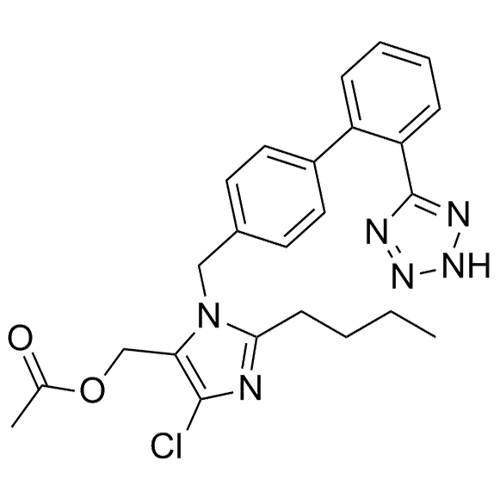
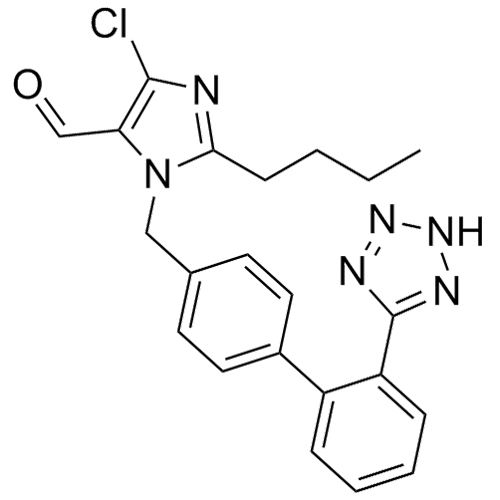
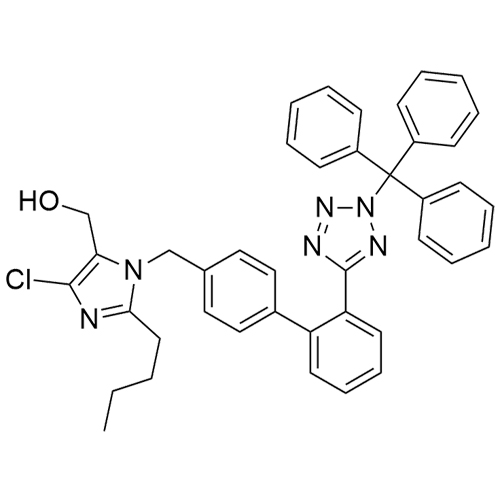
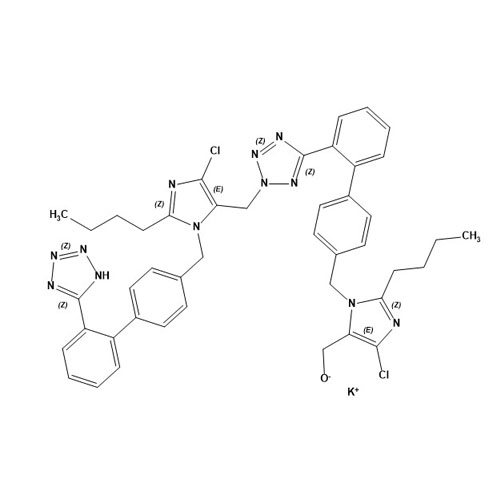
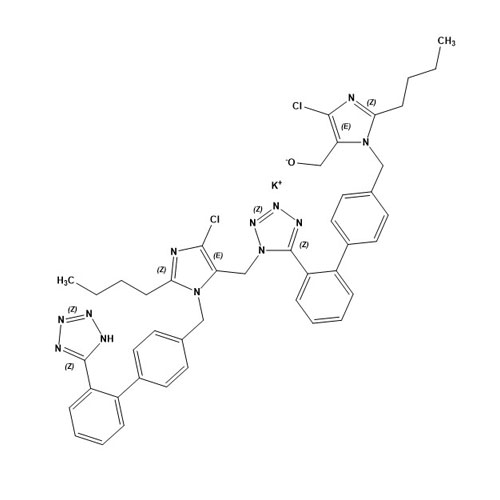
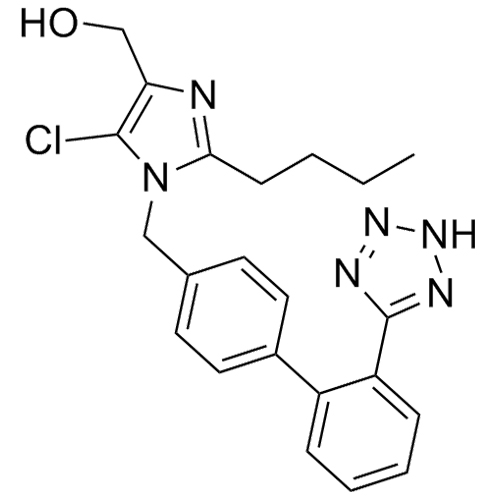
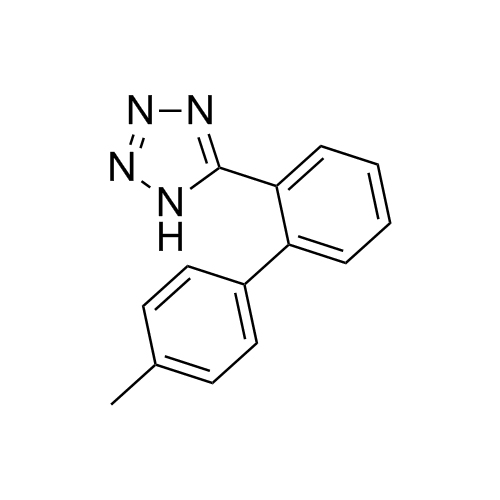
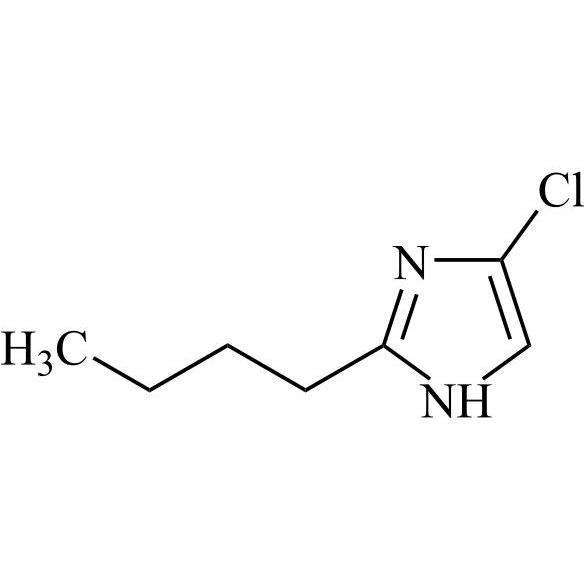
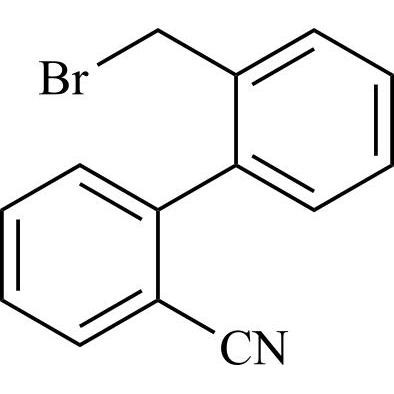
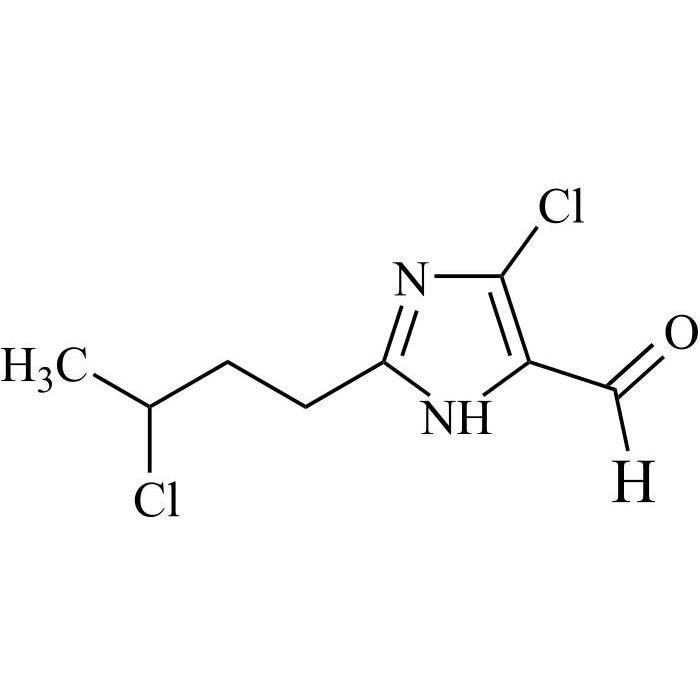
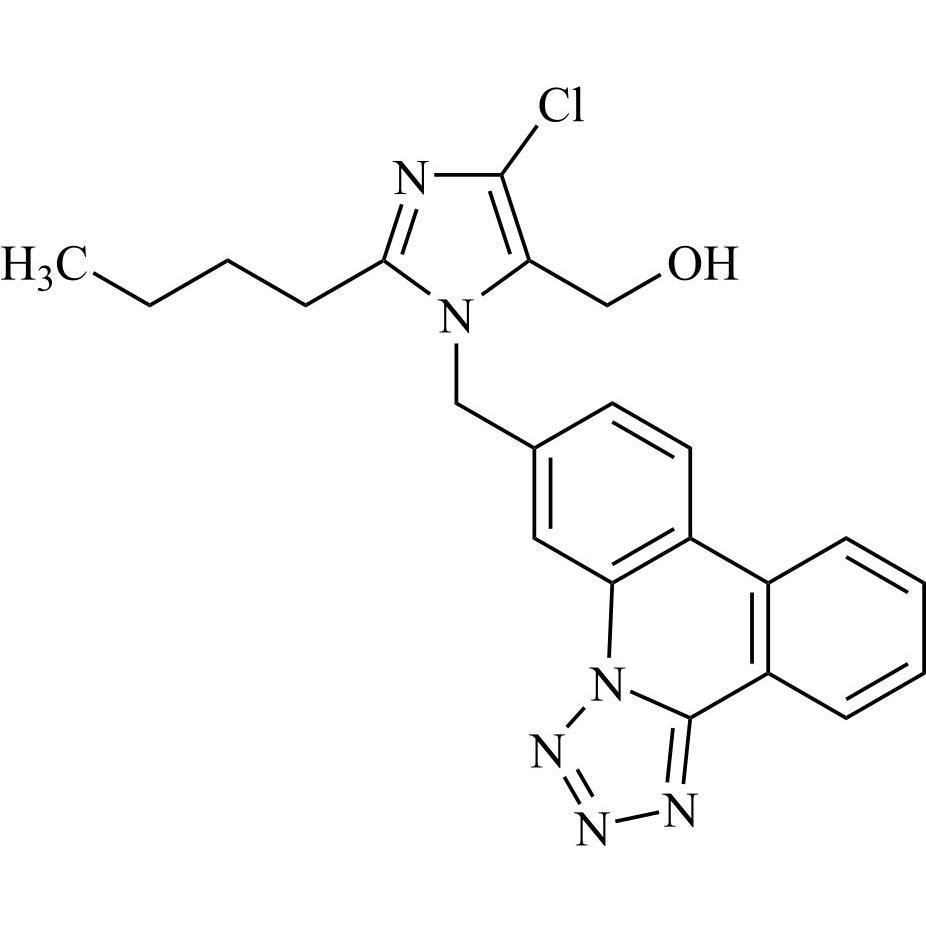
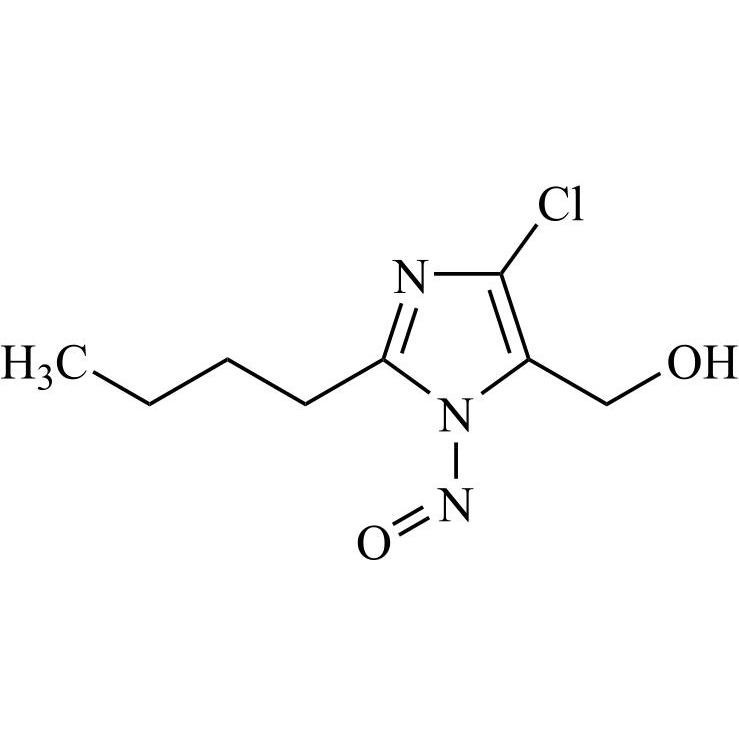
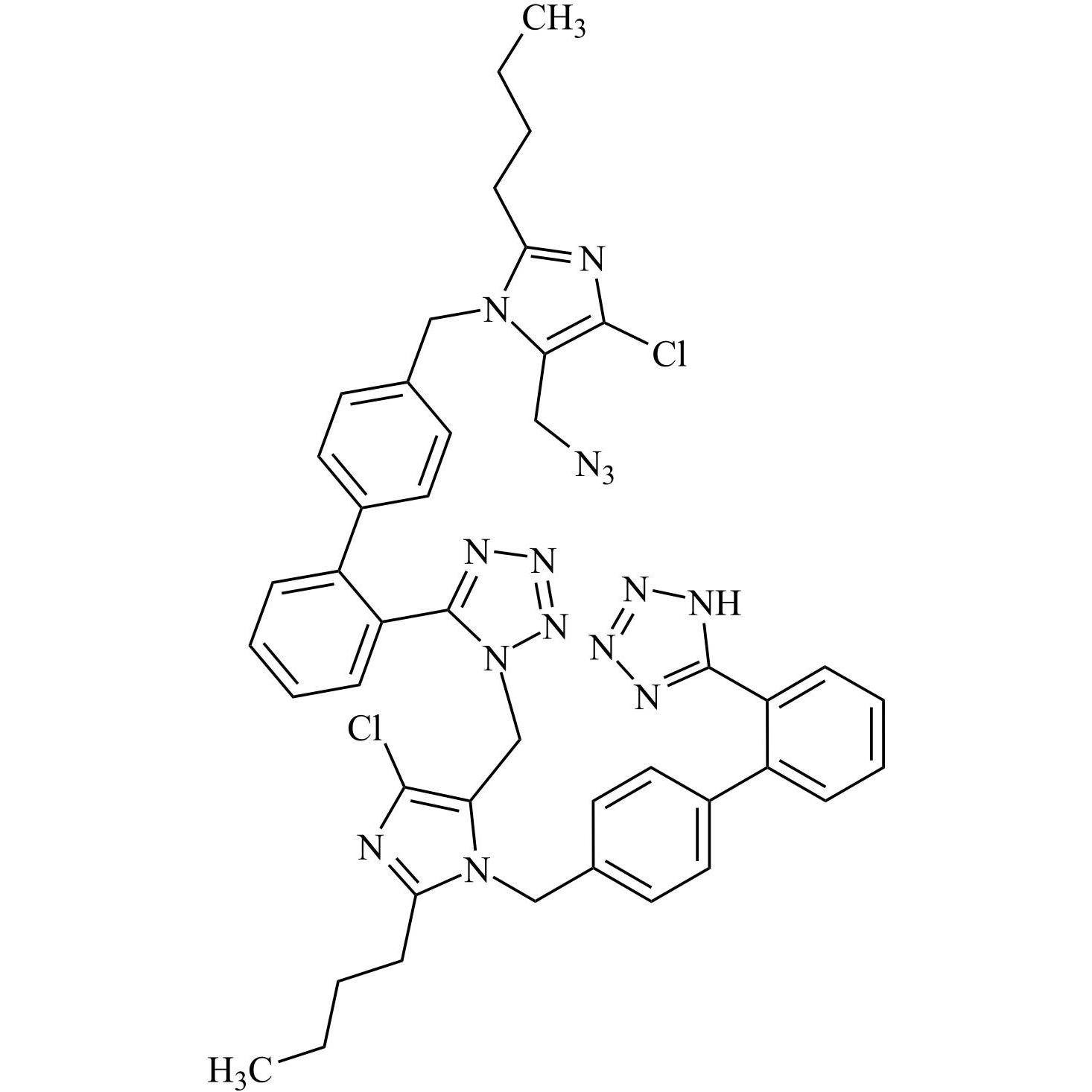
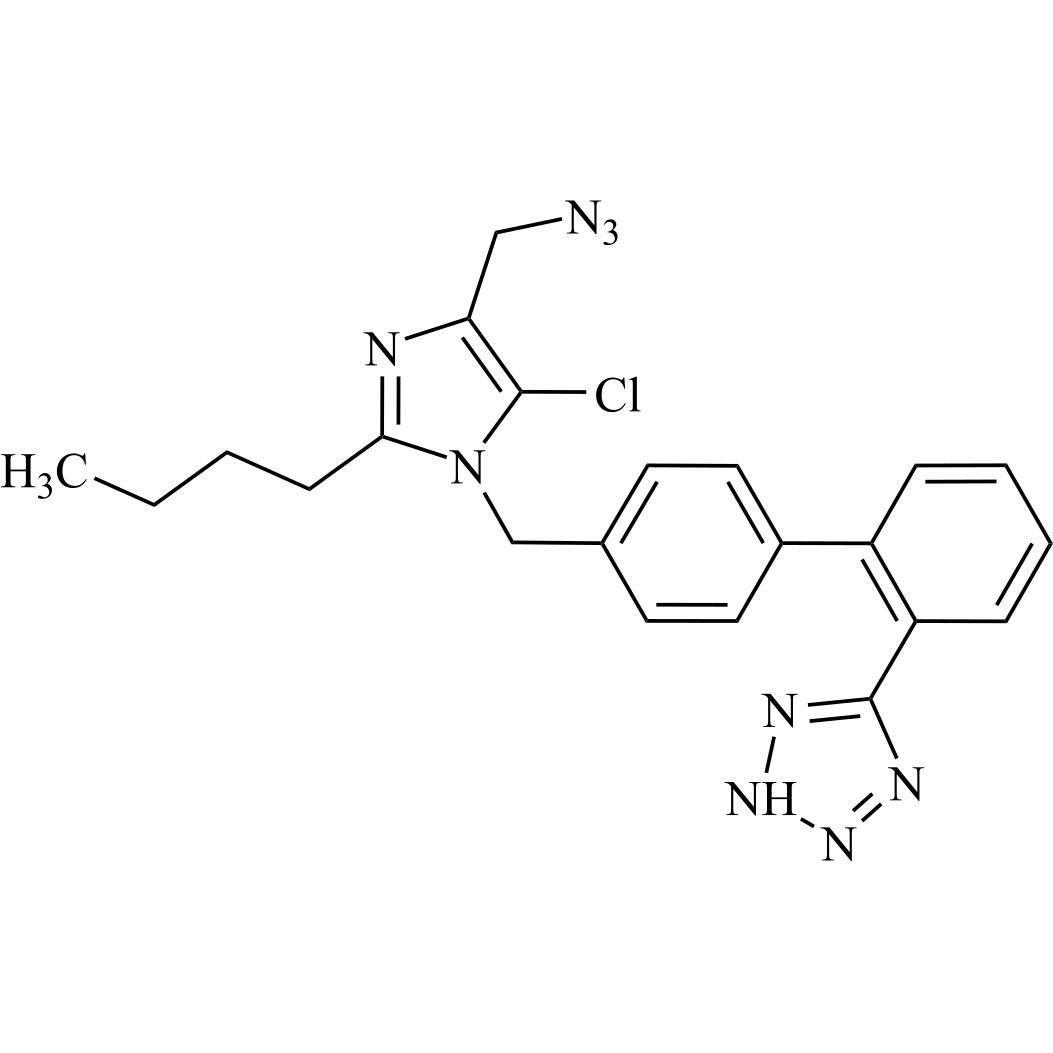
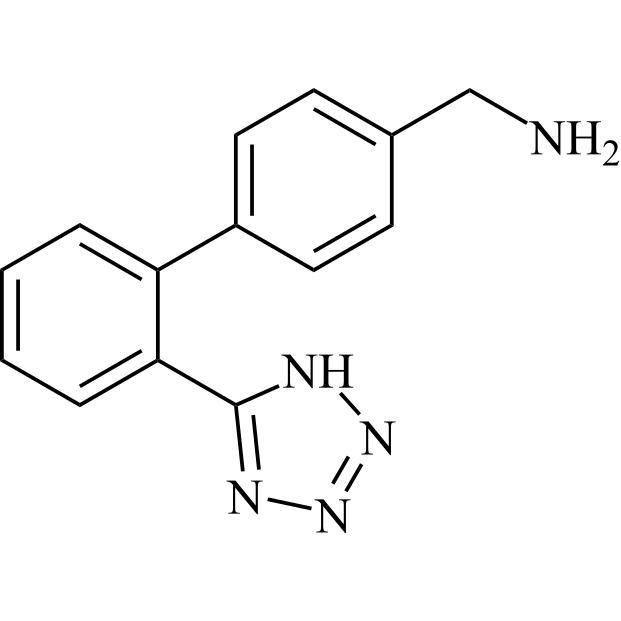
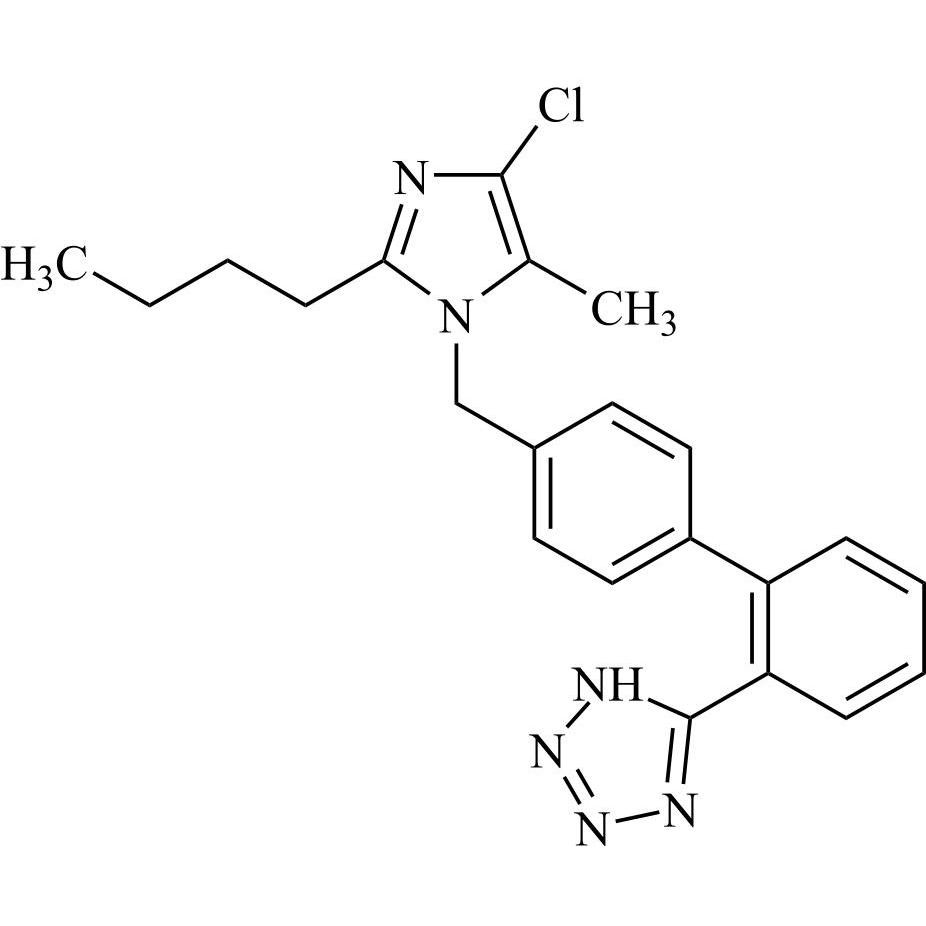
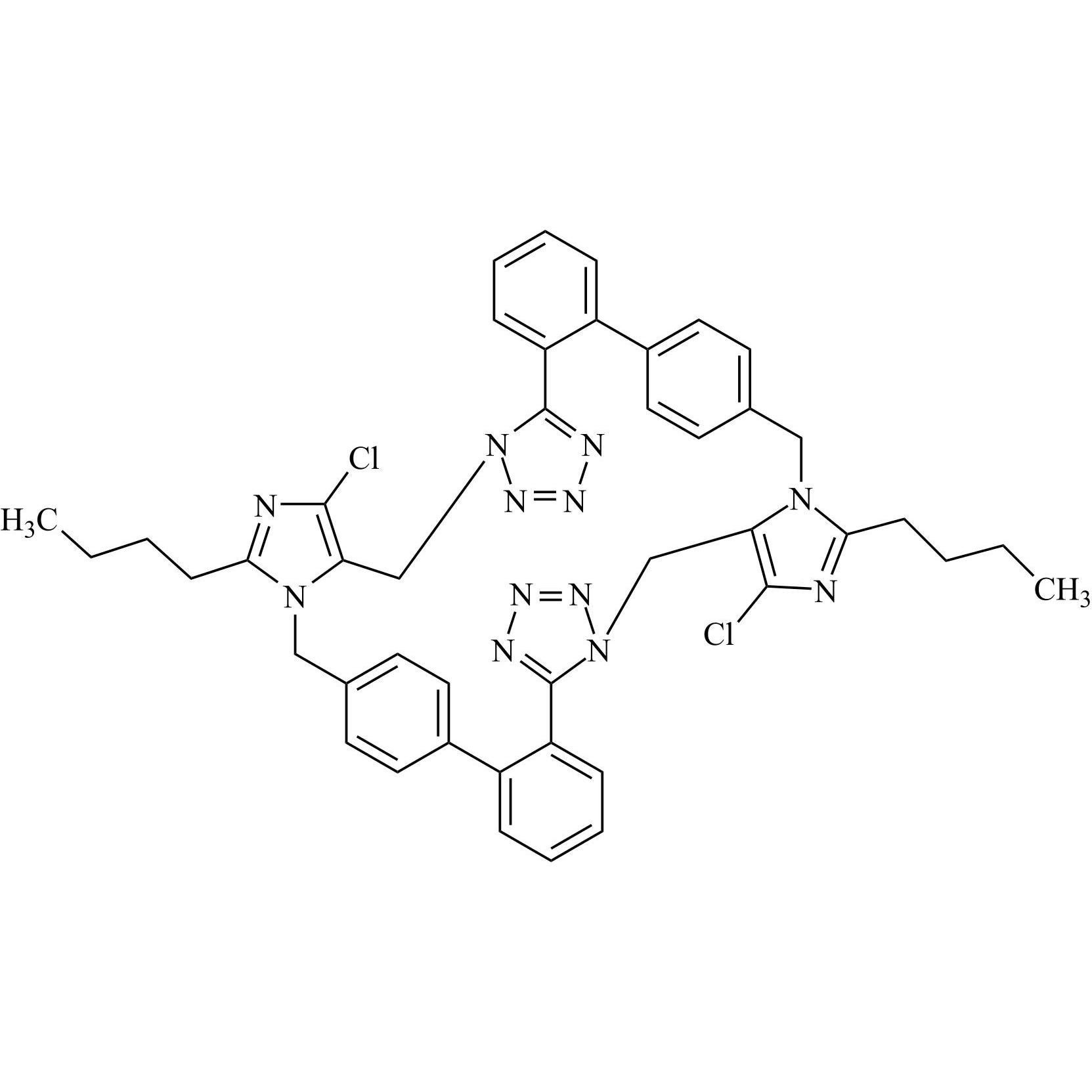
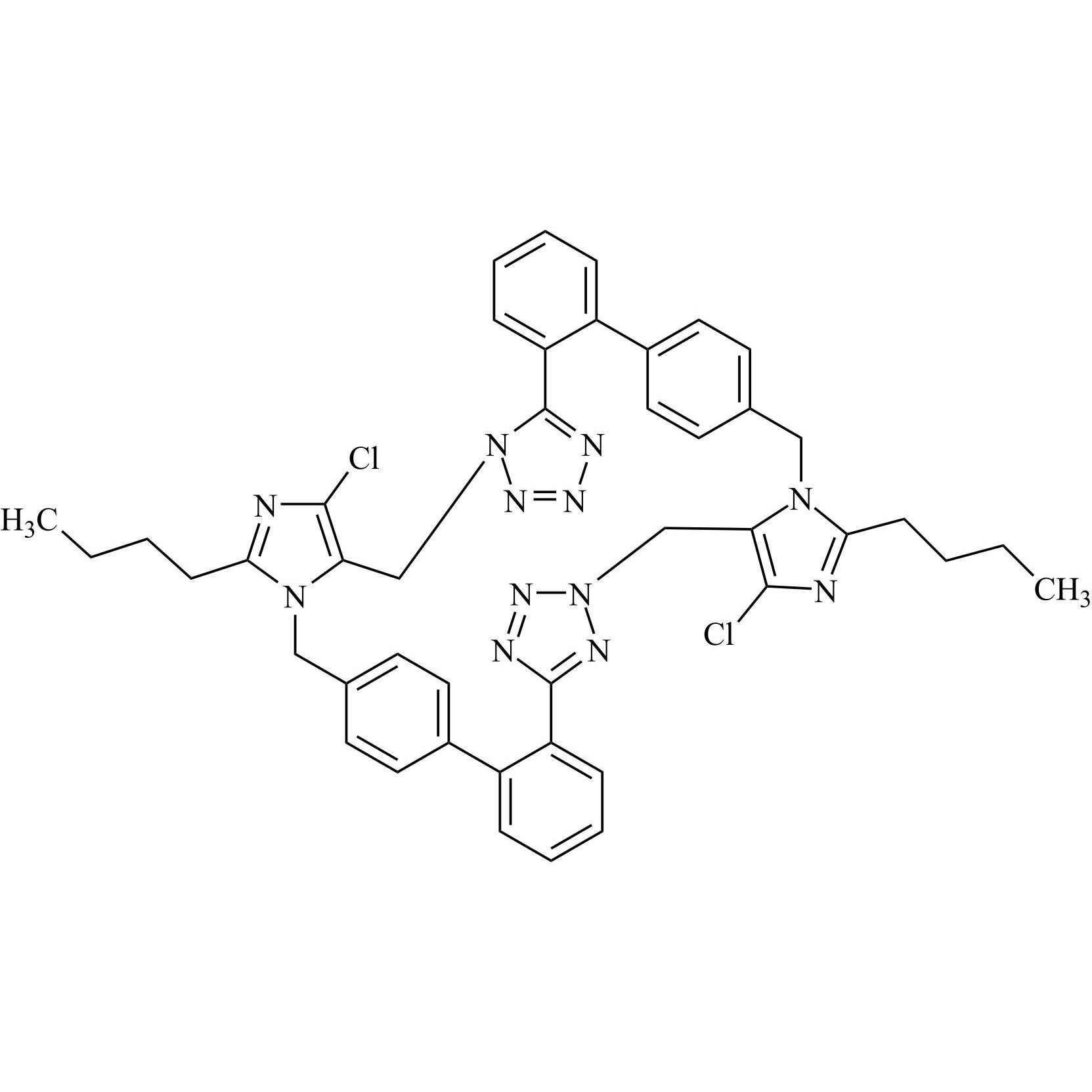
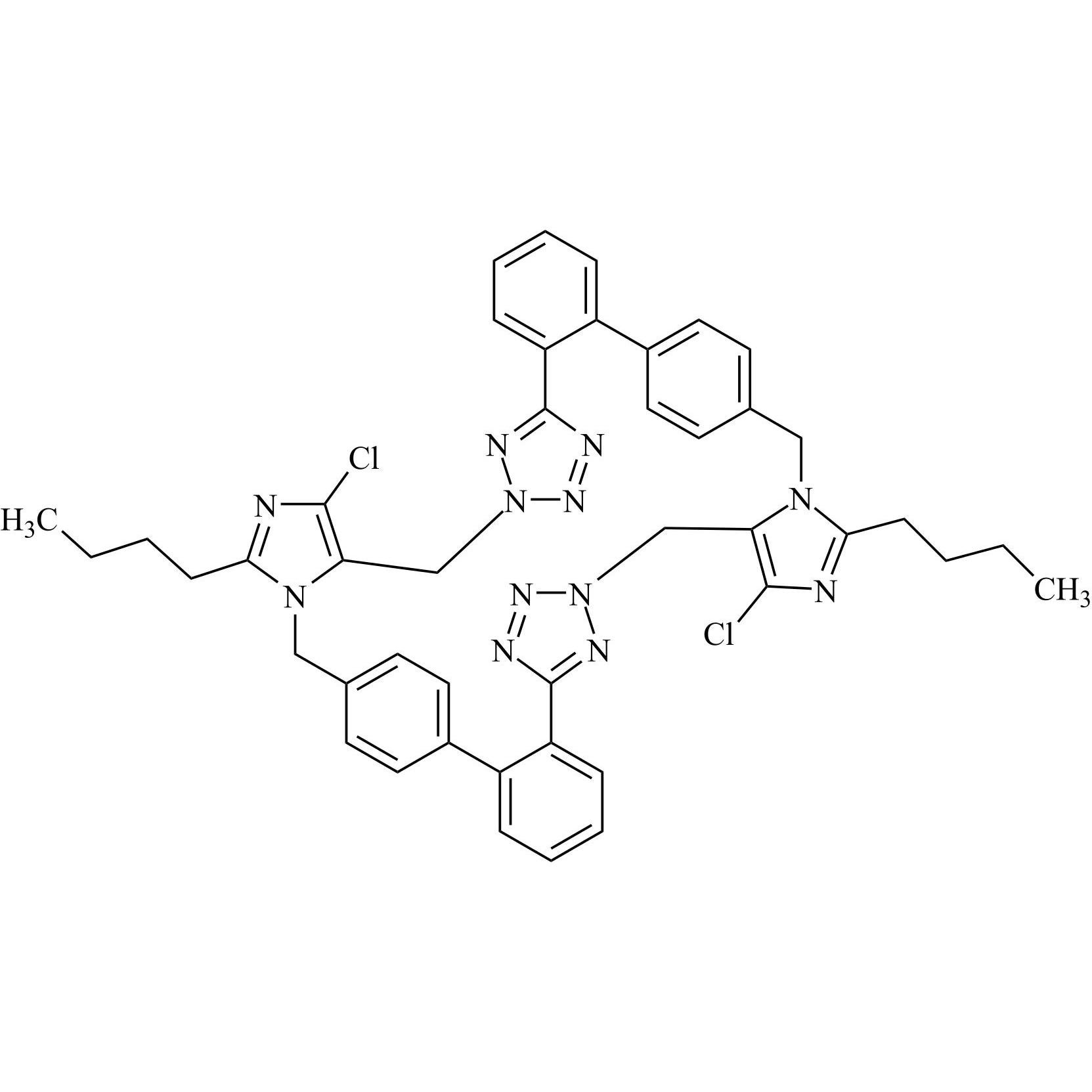
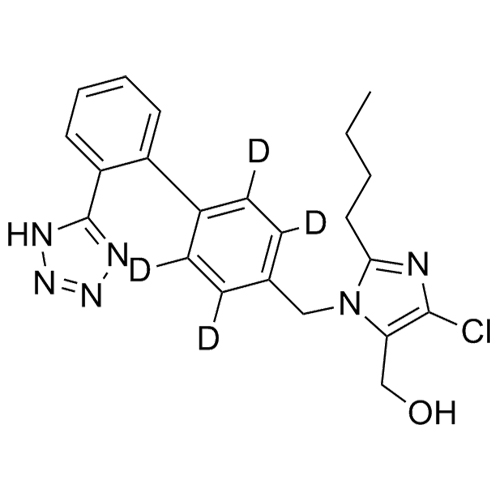
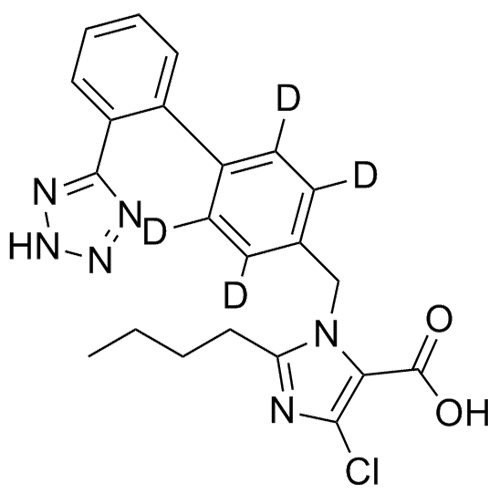
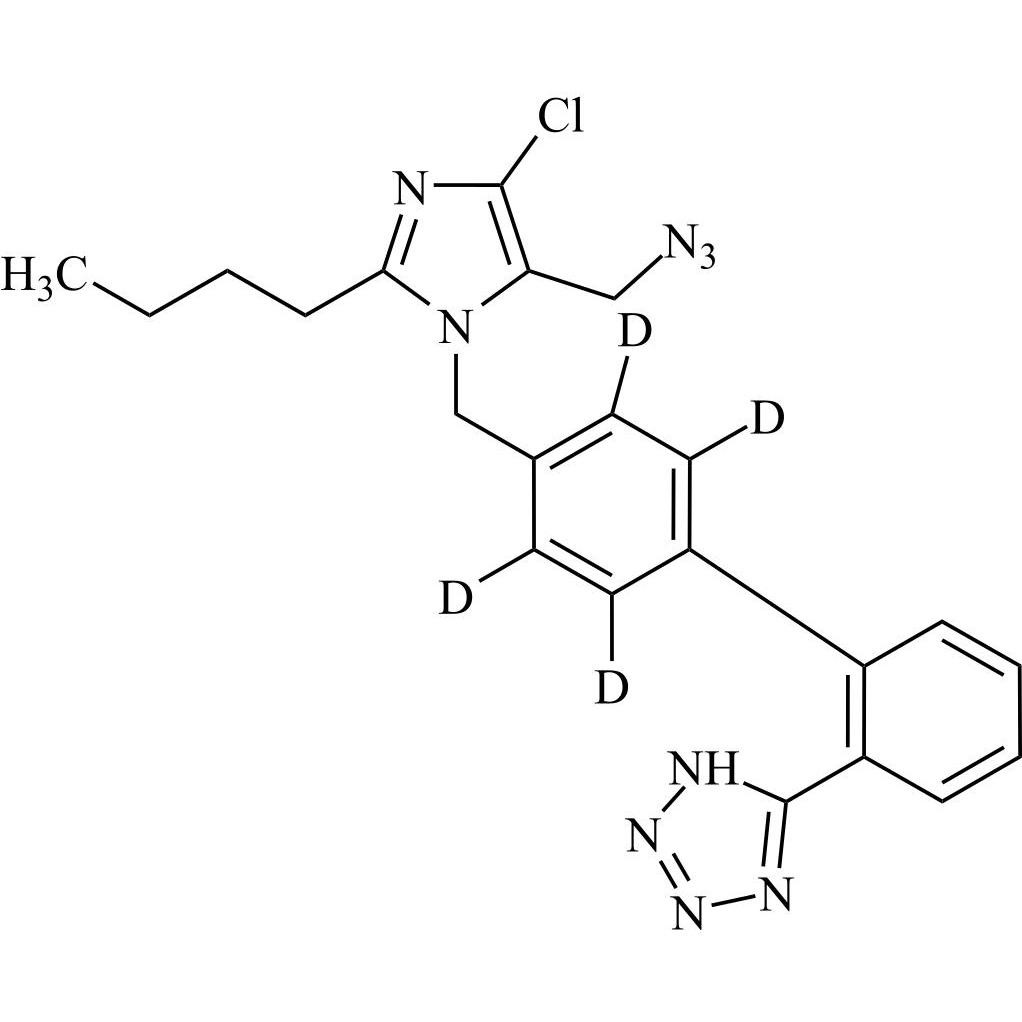
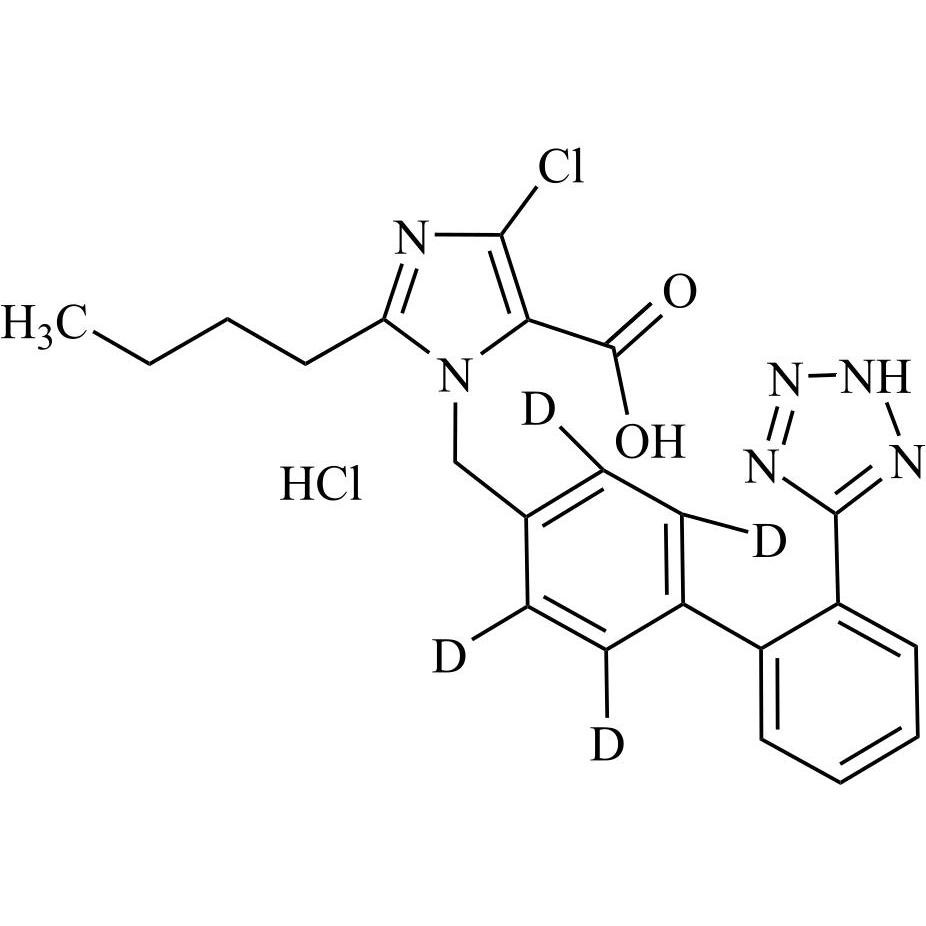
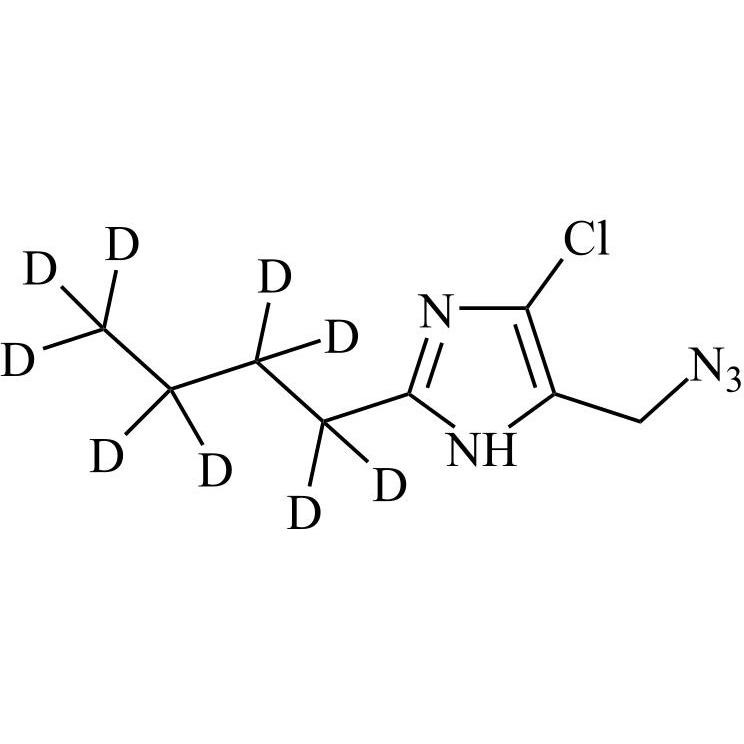
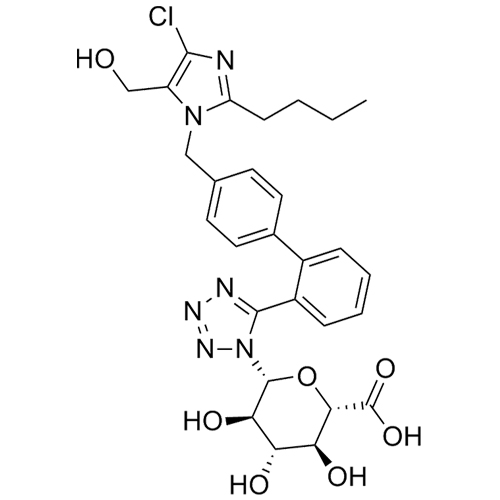
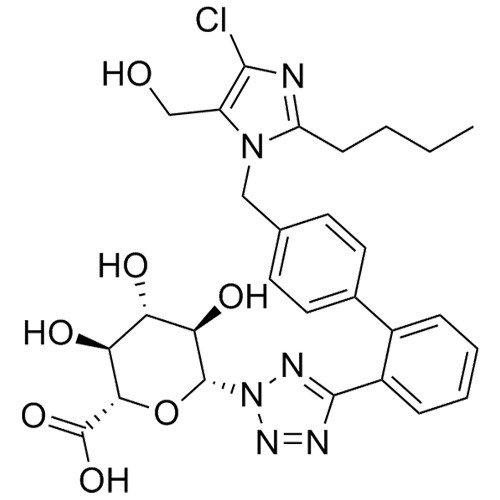
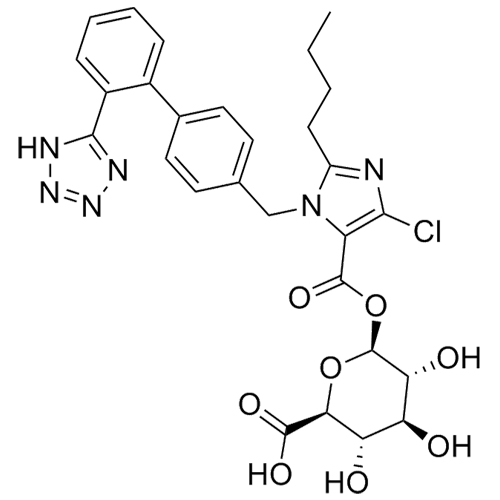
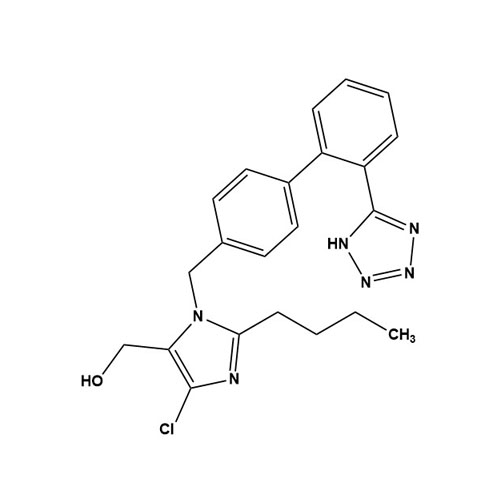
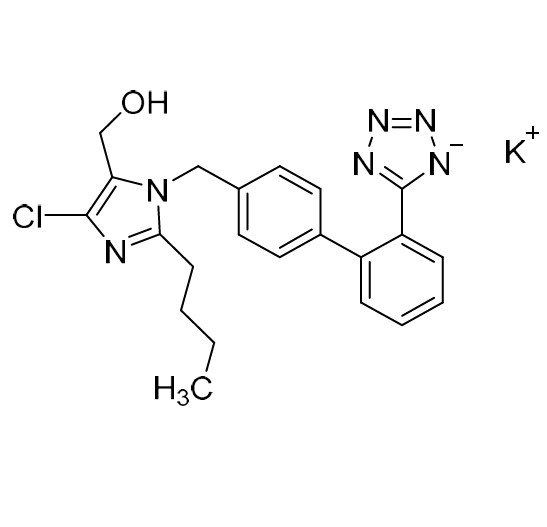
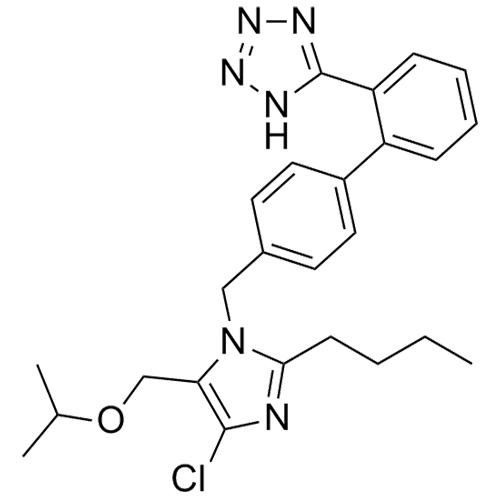
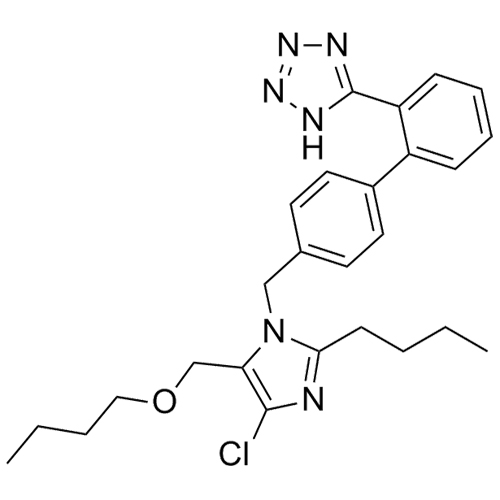
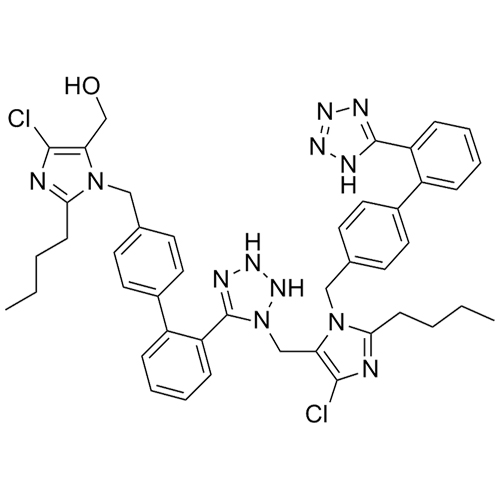
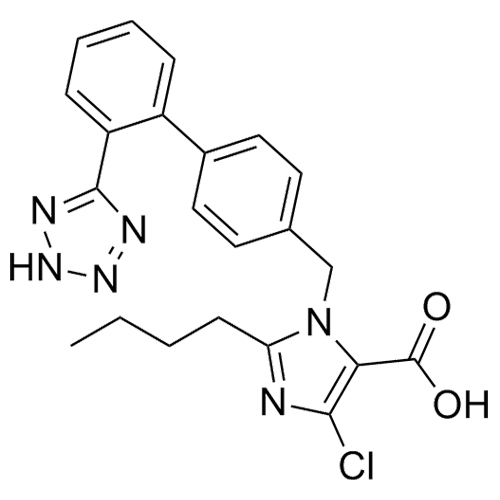
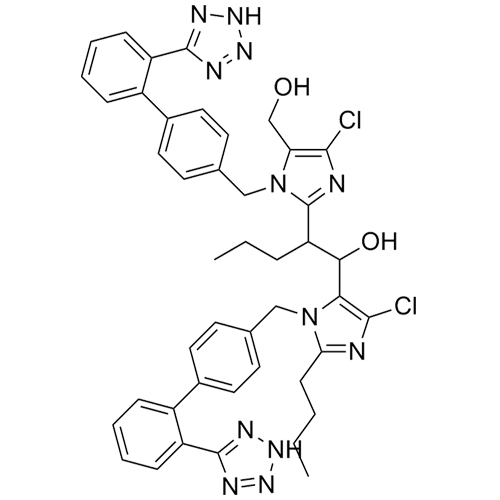
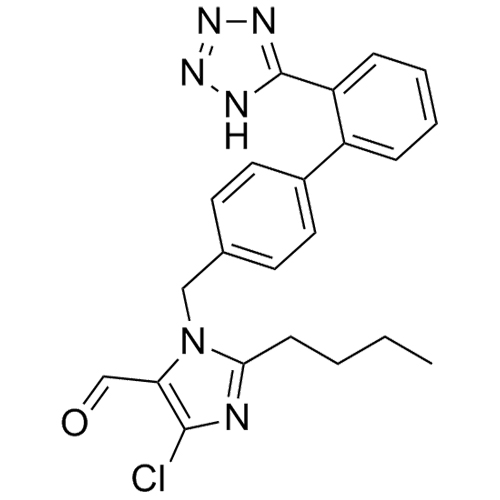
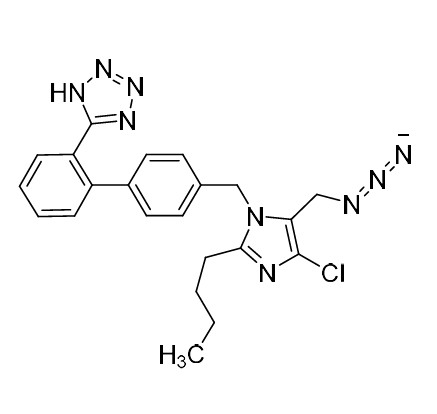
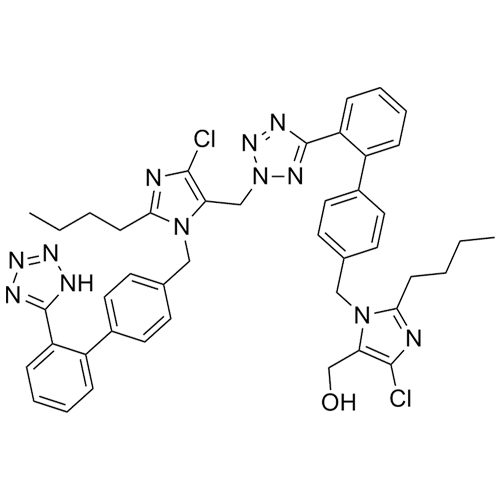
- SynonymsN1-Losartanyl-losartan; 2-Butyl-1-[[2'-[1-[[2-butyl-4-chloro-1-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1H-imidazol-5-yl]methyl]-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-4-chloro-1H-imidazole-5-methanol; Losartan Impurity L; Losartan EP Impurity L; Losartan USP-D
- Description
N1-Losartanyl-losartan; 2-Butyl-1-[[2'-[1-[[2-butyl-4-chloro-1-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-1H-imidazol-5-yl]methyl]-1H-tetrazol-5-yl][1,1'-biphenyl]-4-yl]methyl]-4-chloro-1H-imidazole-5-methanol; Losartan Impurity L; Losartan EP Impurity L; Losartan USP-D
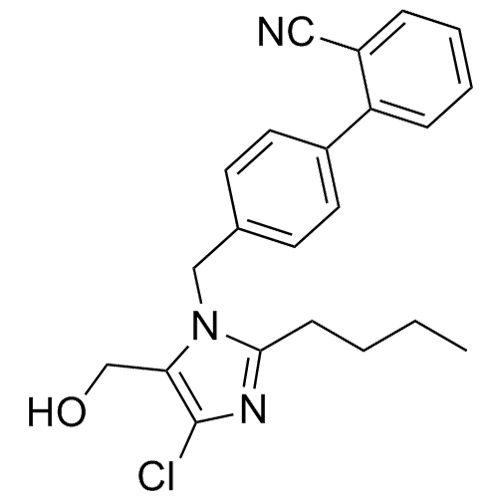
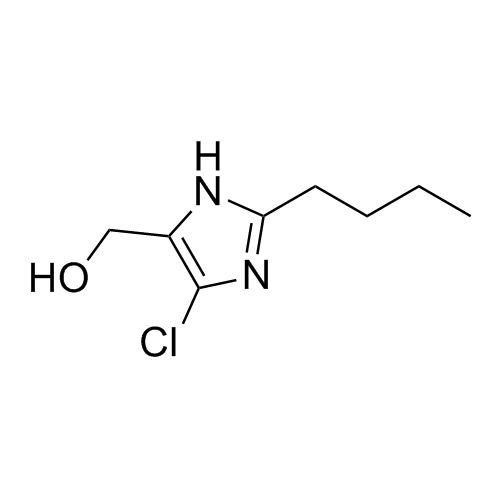
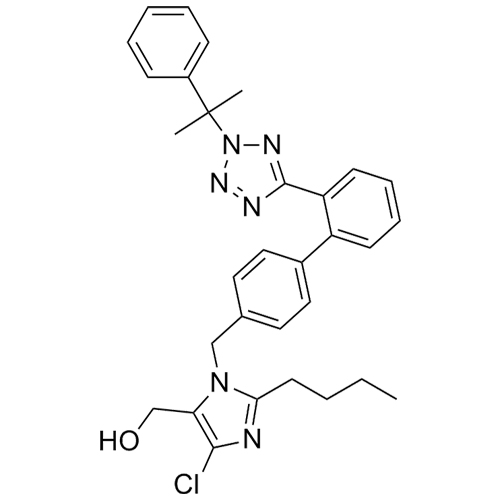
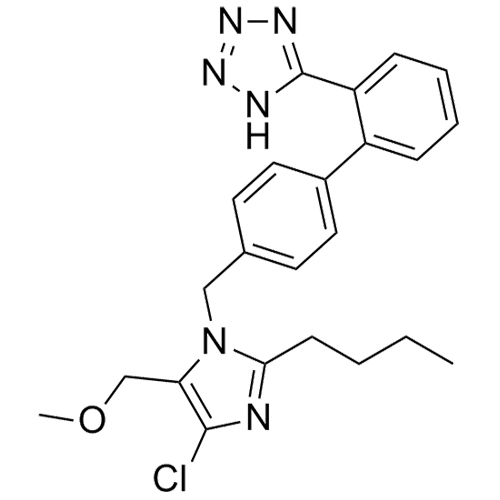
Losartan EP Impurity L is a fully characterized chemical compound used as a reference standard of API Losartan. The standard offered is compliant with regulatory guidelines. Losartan EP Impurity L is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 230971-71-8


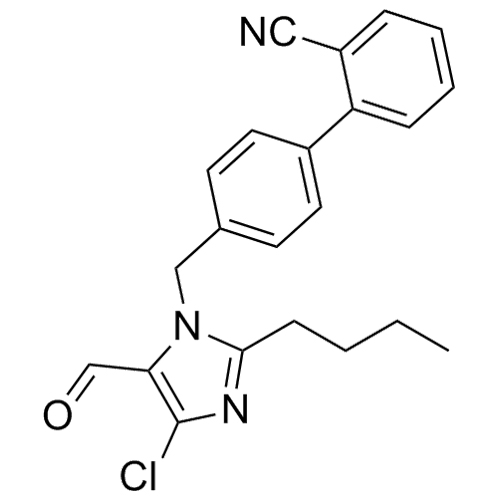
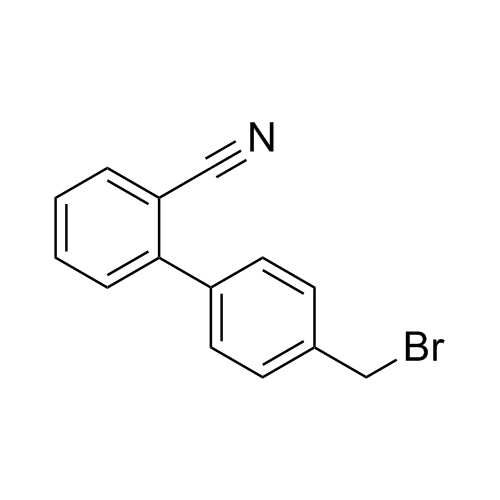
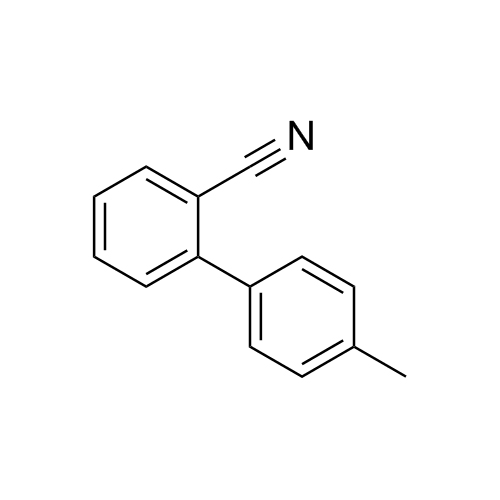
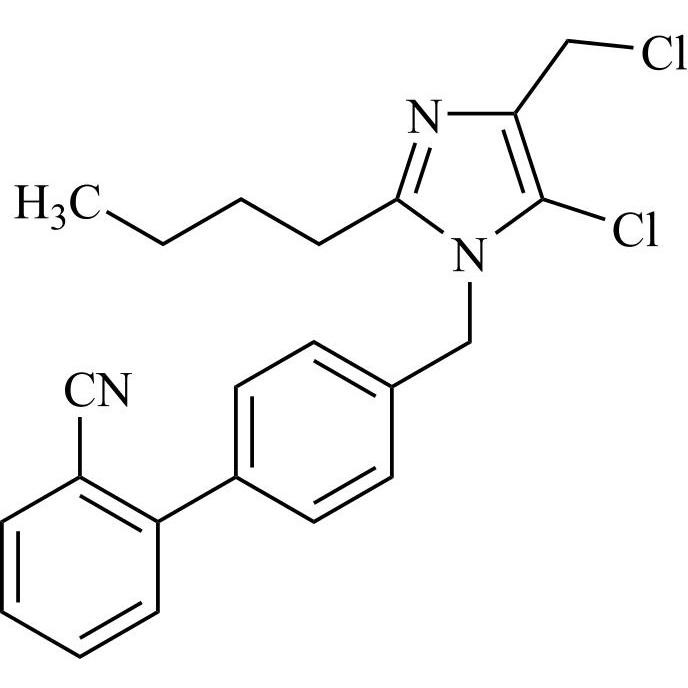
![Show details for Des[2'-(1H-tetrazol-5-yl)] 2-cyanolosartan Picture of Des[2'-(1H-tetrazol-5-yl)] 2-cyanolosartan](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-B17902.jpg?size=256)