- Synonyms4-(((2,4-diaminopteridin-6-yl)methyl)(methyl)amino)benzoicacid; 4-[[(2,4-Diamino-6-pteridinyl)methyl]methylamino]benzoic Acid; 2,4-Diamino-N10- methylpteroic Acid; 4-Amino-4-deoxy-10-methylpteroic Acid; 4-Amino-4-deoxy-N10- methylpteroic Acid; 4-Amino-4-deoxy-N10-methylpteroic Acid; 4-Deoxy-4-ami...
- Description
Related products
7-Hydroxy Methotrexate Trisodium Salt

M.F.
M.W. 467.42 3 22.99
CAT# AR-M01605
CAS# 5939-37-7 (free acid and keto form)
Methotrexate Triglutamate Trifluoroacetate
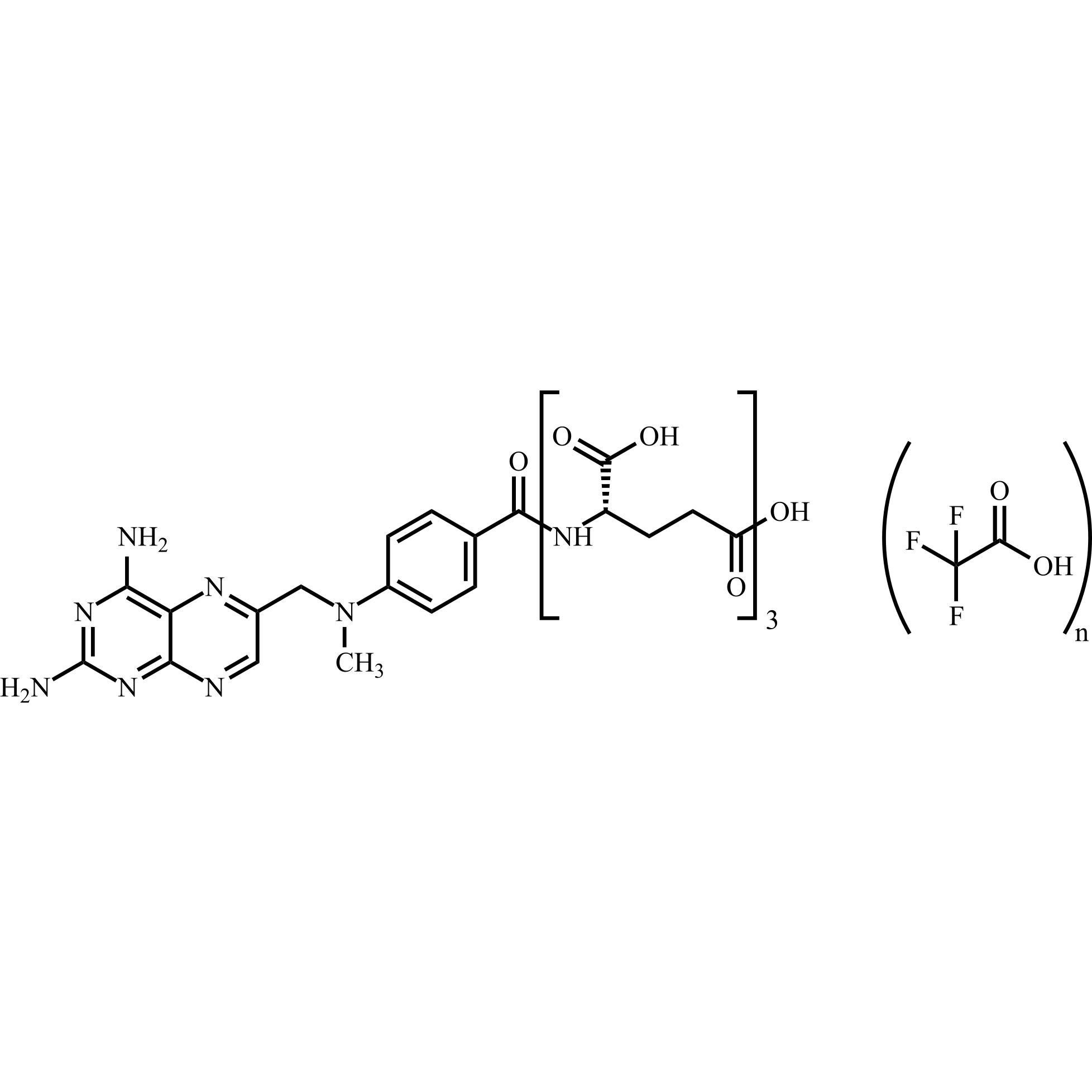
M.F.
M.W. 712.67
CAT# AR-M04426
CAS# 41600-14-0 (free amine)
Methotrexate EP Impurity I Enantiomer ((R)-Methotrexate-1- Monomethyl Ester)

M.F.
M.W. 468.47
CAT# AR-M04457
CAS# 440339-37-7
Methotrexate Heptaglutamate Trifluoroacetate

M.F.
M.W. 1229.14
CAT# AR-M04430
CAS# 59-05-2 (free amine)
Methotrexate Tetraglutamate Trifluoroacetate

M.F.
M.W. 841.78
CAT# AR-M04428
CAS# 73610-81-8 (free amine)
N-[4-[[(2-amino-6-pteridinyl)methyl]amino]benzoyl]-Glutamic acid
![Show details for N-[4-[[(2-amino-6-pteridinyl)methyl]amino]benzoyl]-Glutamic acid Picture of N-[4-[[(2-amino-6-pteridinyl)methyl]amino]benzoyl]-Glutamic acid](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-M04432.jpg?size=256)
M.F.
M.W. 425.41
CAT# AR-M04432
CAS# 728946-34-7
Methotrexate EP Impurity F Disodium Salt ((R)-Methotrexate Disodium Salt)
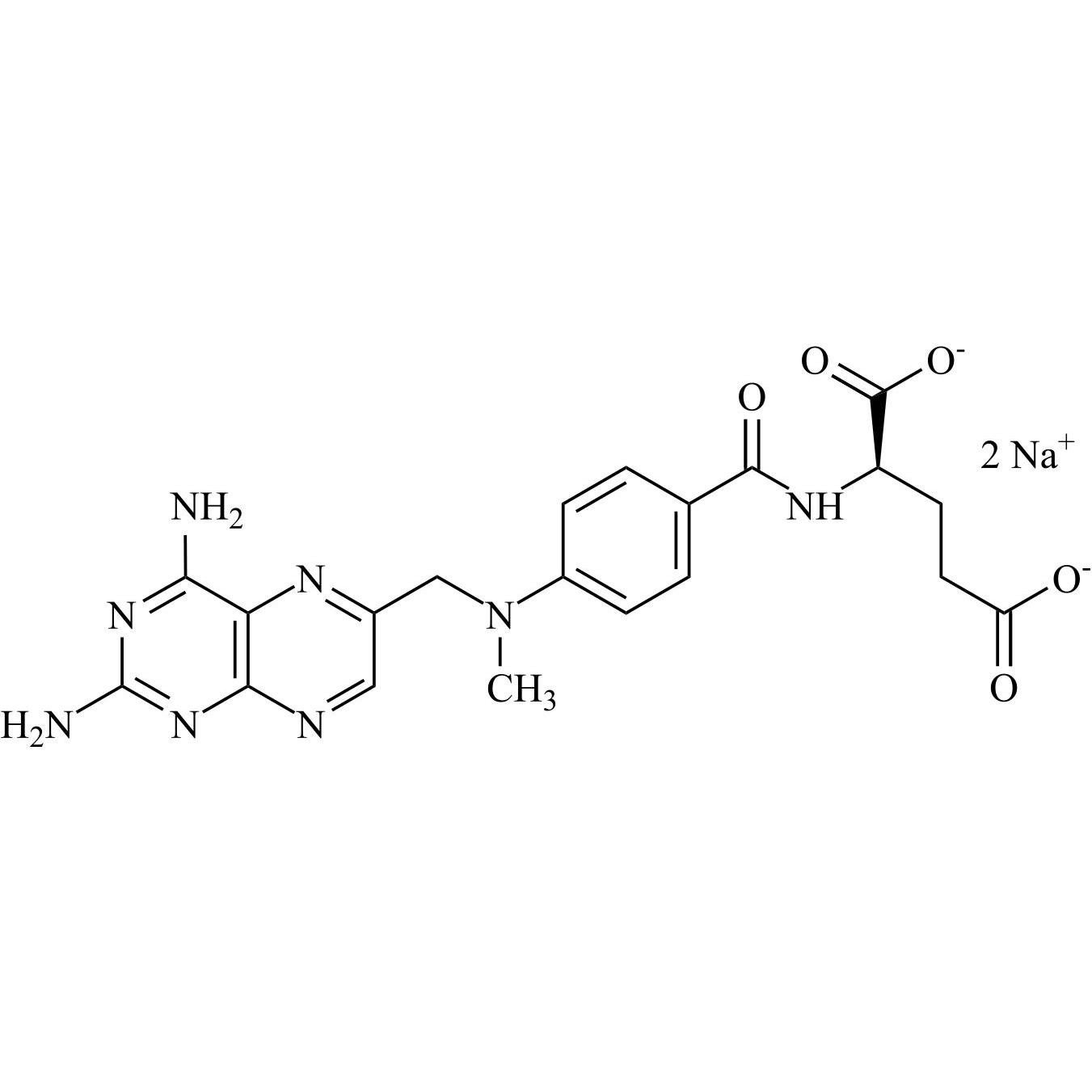
M.F.
M.W. 454.45 2*22.99
CAT# AR-M04448
CAS# NA
Methotrexate EP Impurity I Sodium Salt (Methotrexate-1-Monomethyl Ester Sodium Salt)

M.F.
M.W. 467.47 22.99
CAT# AR-M04452
CAS# NA
Methotrexate-d3 Diglutamate Trifluoroacetate

M.F.
M.W. 586.57
CAT# AR-M04425
CAS# 432545-63-6 (free amine)
Methotrexate-d3 Triglutamate Trifluoroacetate

M.F.
M.W. 715.69
CAT# AR-M04427
CAS# 432545-63-6 (free amine)
Methotrexate-d3 Tetraglutamate Trifluoroacetate

M.F.
M.W. 844.80
CAT# AR-M04429
CAS# 432545-63-6 (free amine)
Methotrexate-d3 Heptaglutamate Trifluoroacetate
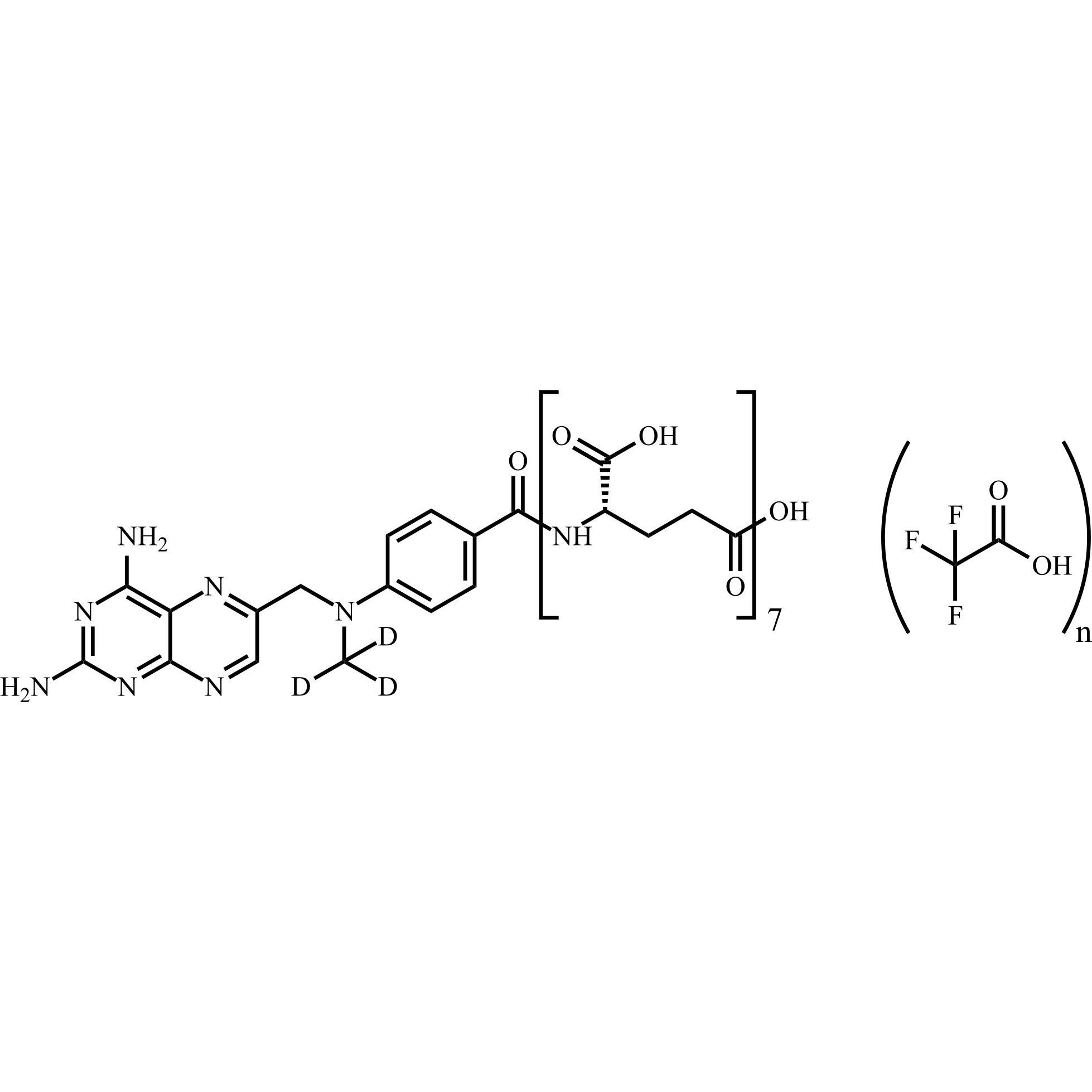
M.F.
M.W. 1232.14
CAT# AR-M04431
CAS# 432545-63-6 (free amine)
Methotrexate EP Impurity F-13C-d3 Disodium Salt ((R)-Methotrexate-13C-d3 Disodium Salt)

M.F.
M.W. 456.44 2*22.99
CAT# AR-M04456
CAS# NA





















































