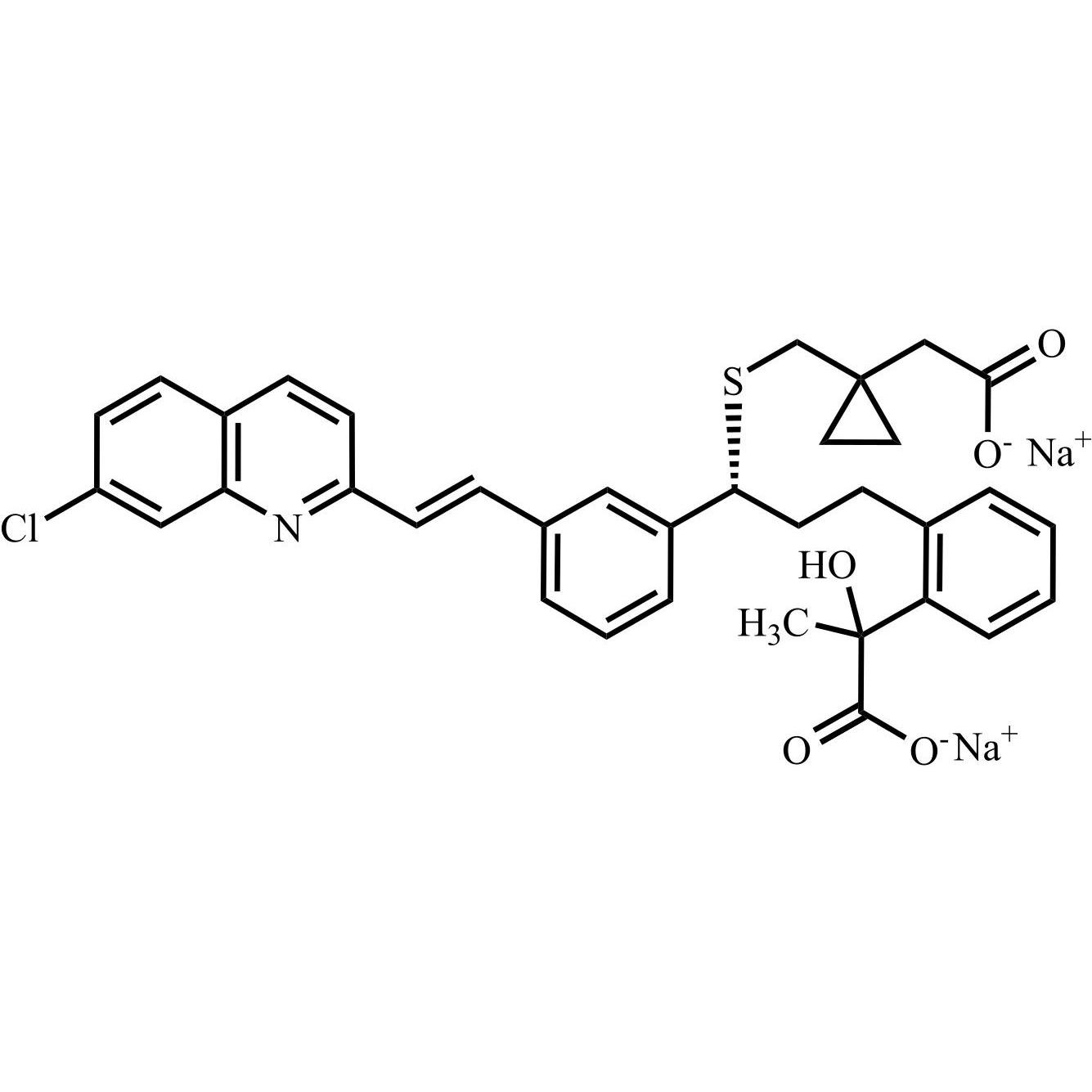
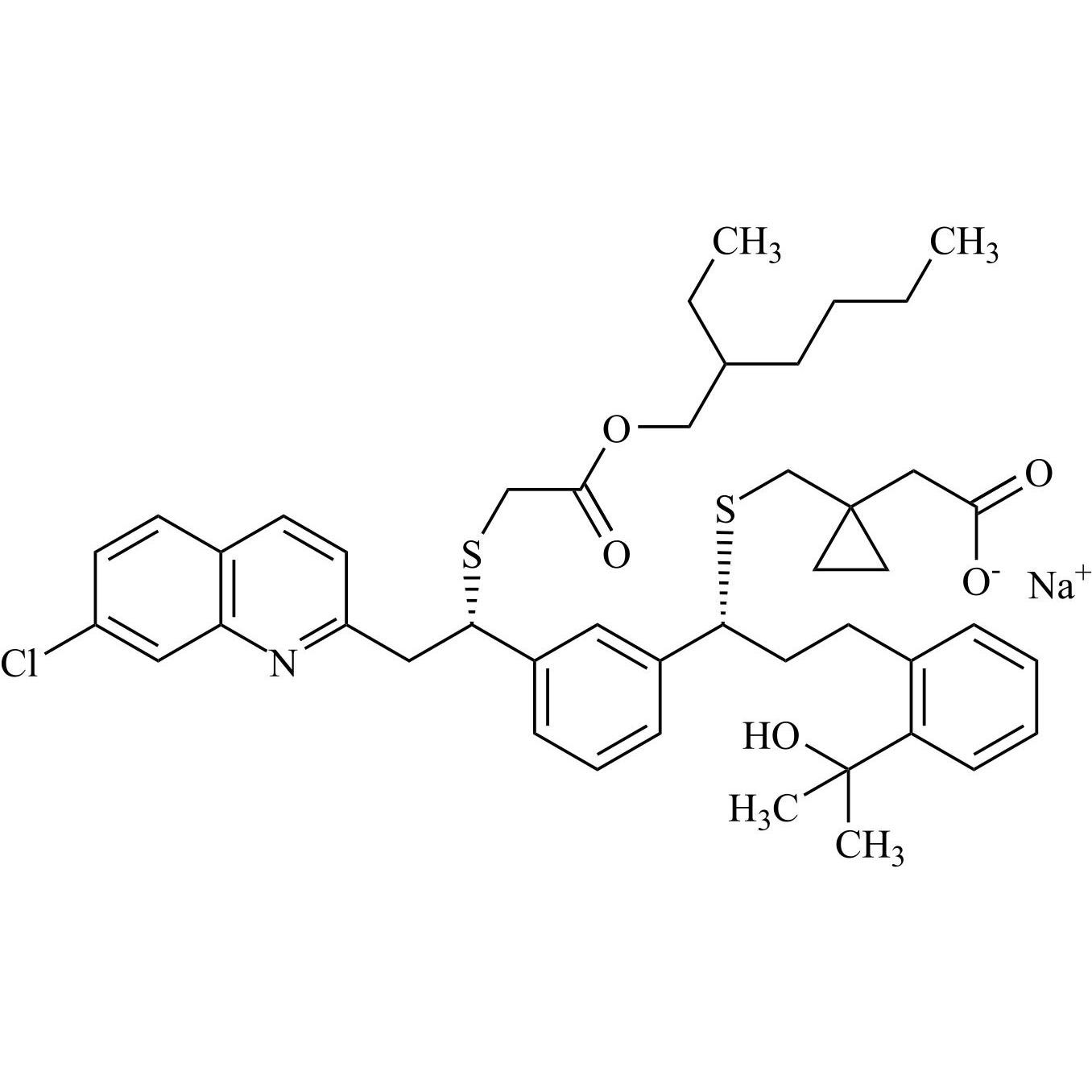
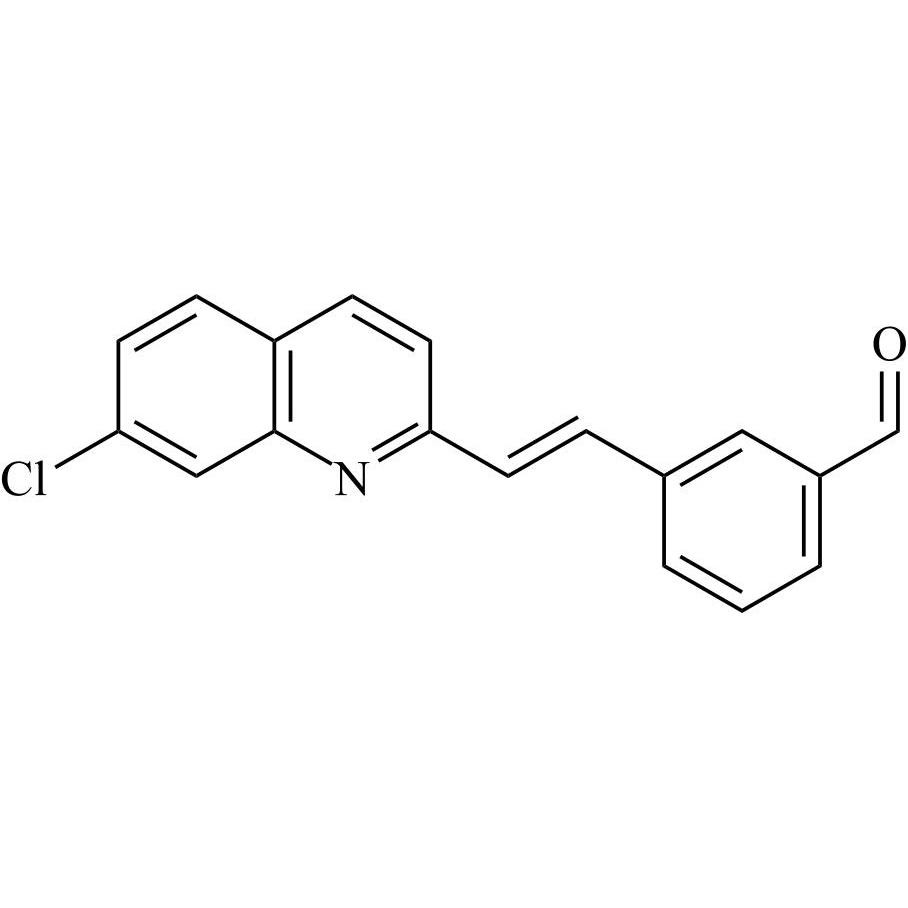
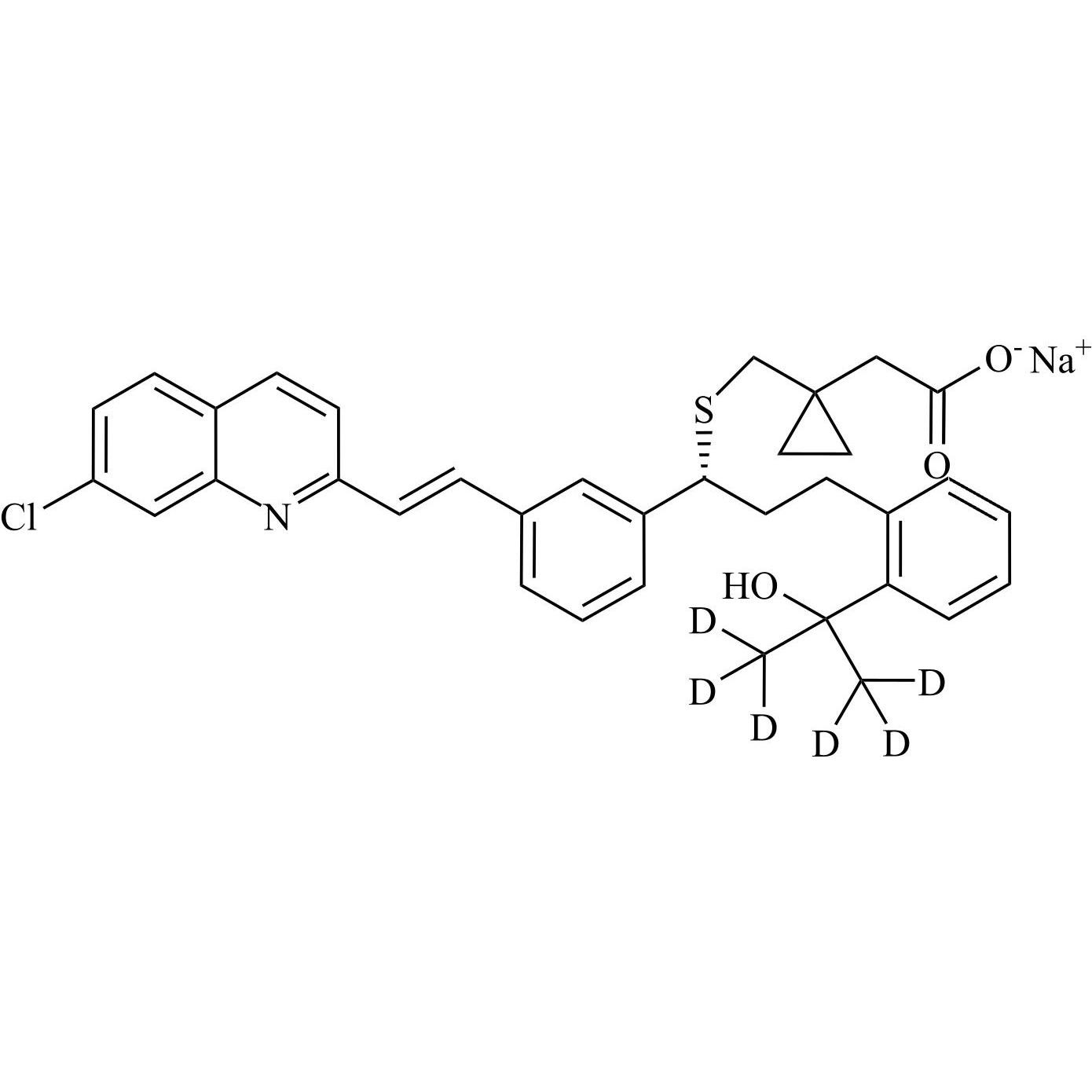
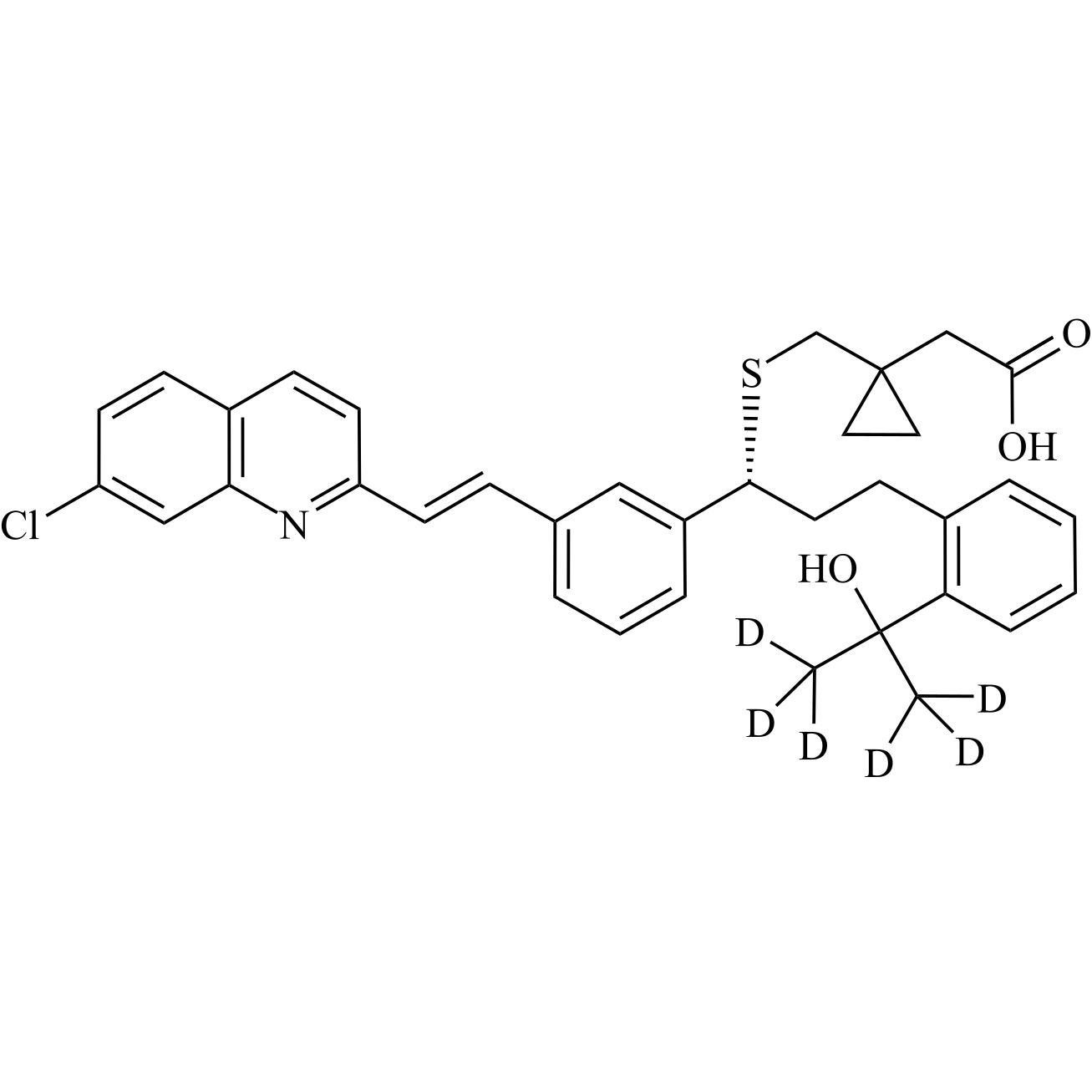
- Synonyms[1-[[[(1R)-1-[3-[(Z)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl]acetic acid sodium salt
- Description
[1-[[[(1R)-1-[3-[(Z)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]sulfanyl]methyl]cyclopropyl]acetic acid sodium salt
Montelukast EP Impurity G Sodium Salt is a fully characterized chemical compound used as a reference standard of API Montelukast. The standard offered is compliant with regulatory guidelines. Montelukast EP Impurity G Sodium Salt is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 1428448-96-7
Related products
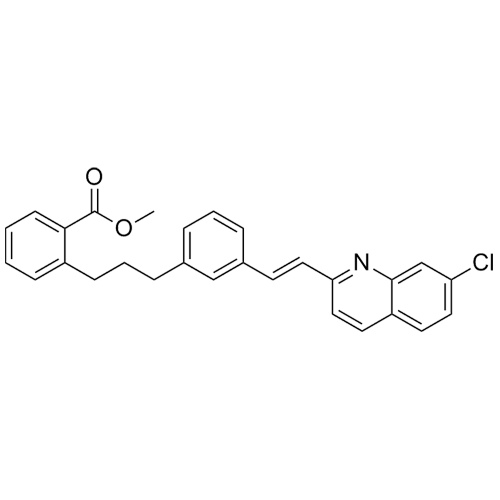
Montelukast methyl methanesulfonate fragment Impurity

M.F.
M.W. 189.2
CAT# AR-M03441
CAS# 152922-86-6
Montelukast (3S)-Hydroxy Benzoate with TBC as stabilizer
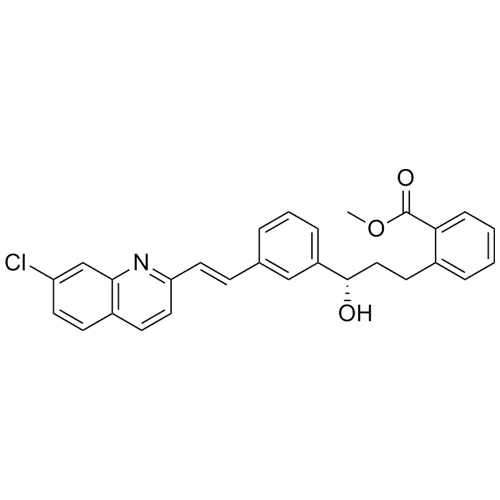
M.F.
M.W. 457.96
CAT# AR-M02247
CAS# 142569-69-5
Montelukast R,S-Isomer (Montelukast EP Impurity E)

M.F.
M.W. 732.39
CAT# AR-M02230
CAS# 1187586-58-8
Montelukast EP Impurity D and Montelukast EP Impurity E (Mixture of Diastereomers)
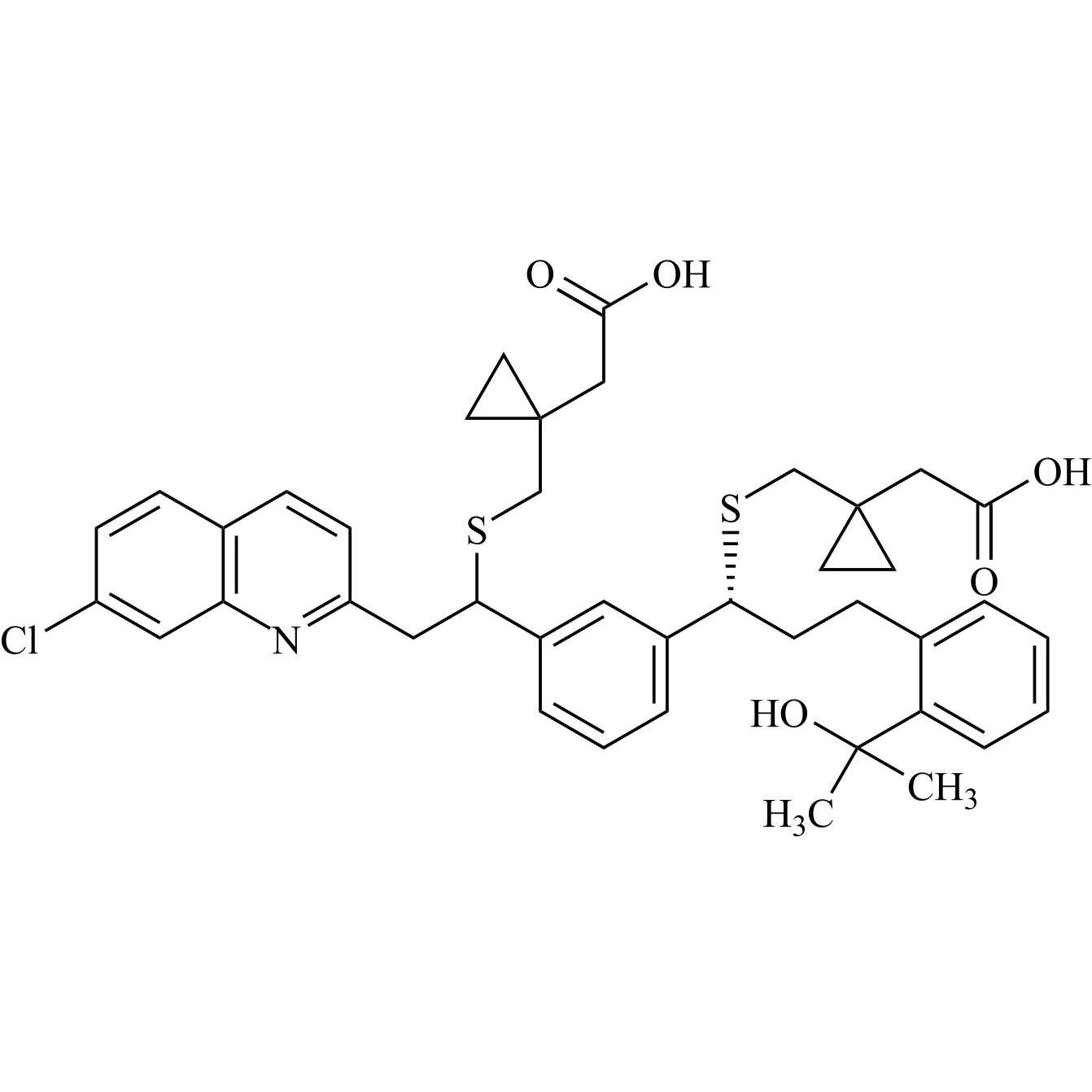
M.F.
M.W. 732.39
CAT# AR-M05077
CAS# 1242260-05-4
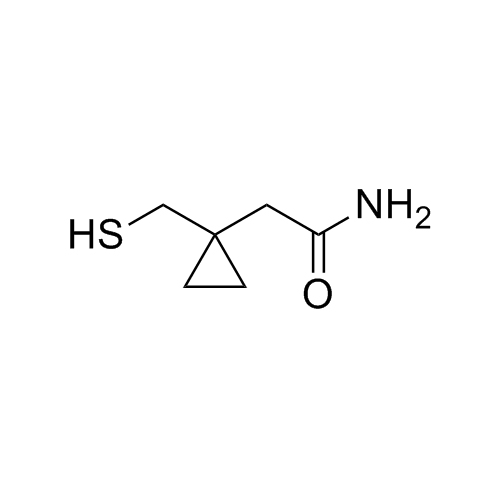
Montelukast EP Impurity I Sodium Salt (Mixture of Diastereomers)
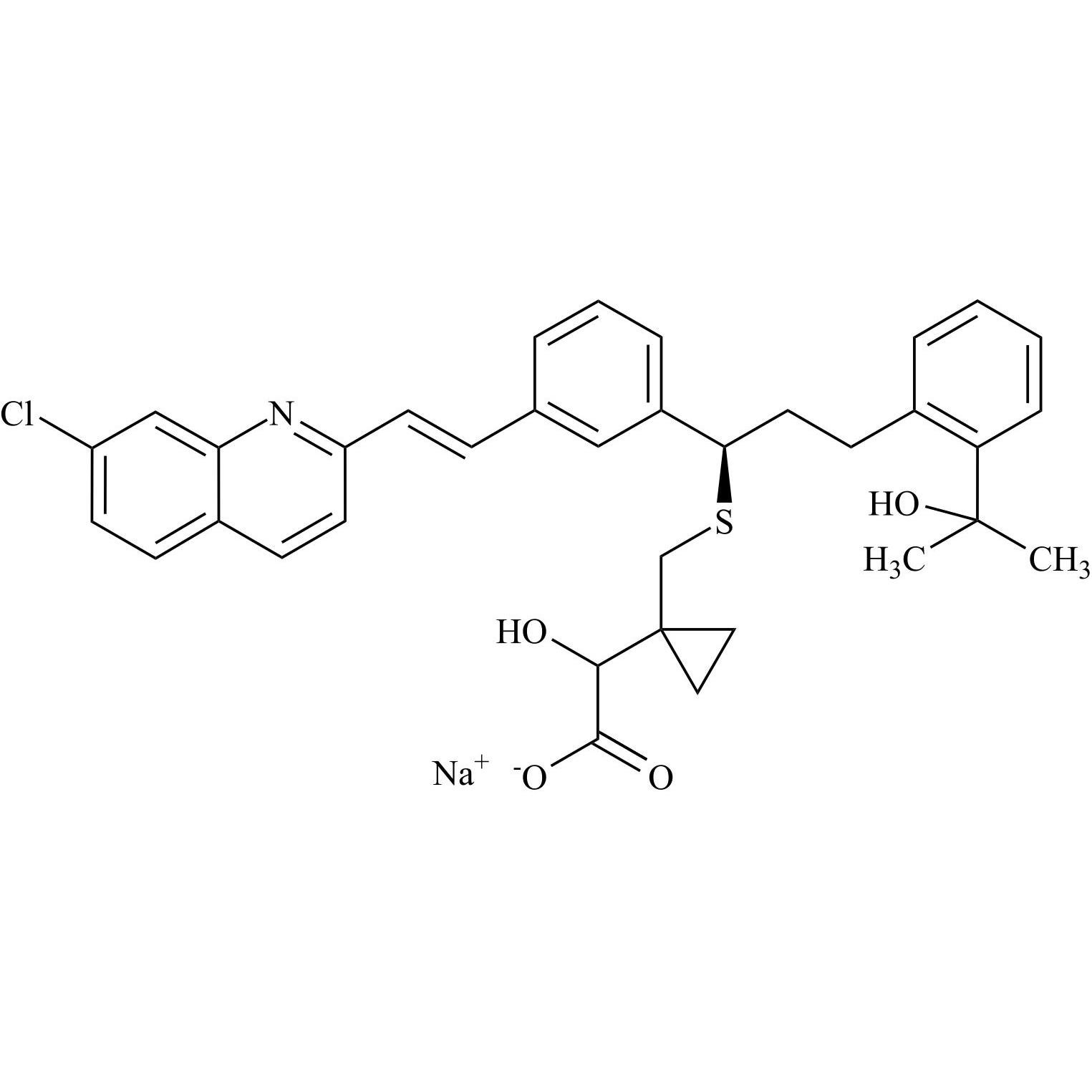
M.F.
M.W. 601.18 22.99
CAT# AR-M05073
CAS# NA
rac-Montelukast EP Impurity F (rac-Montelukast Methyl Ketone)
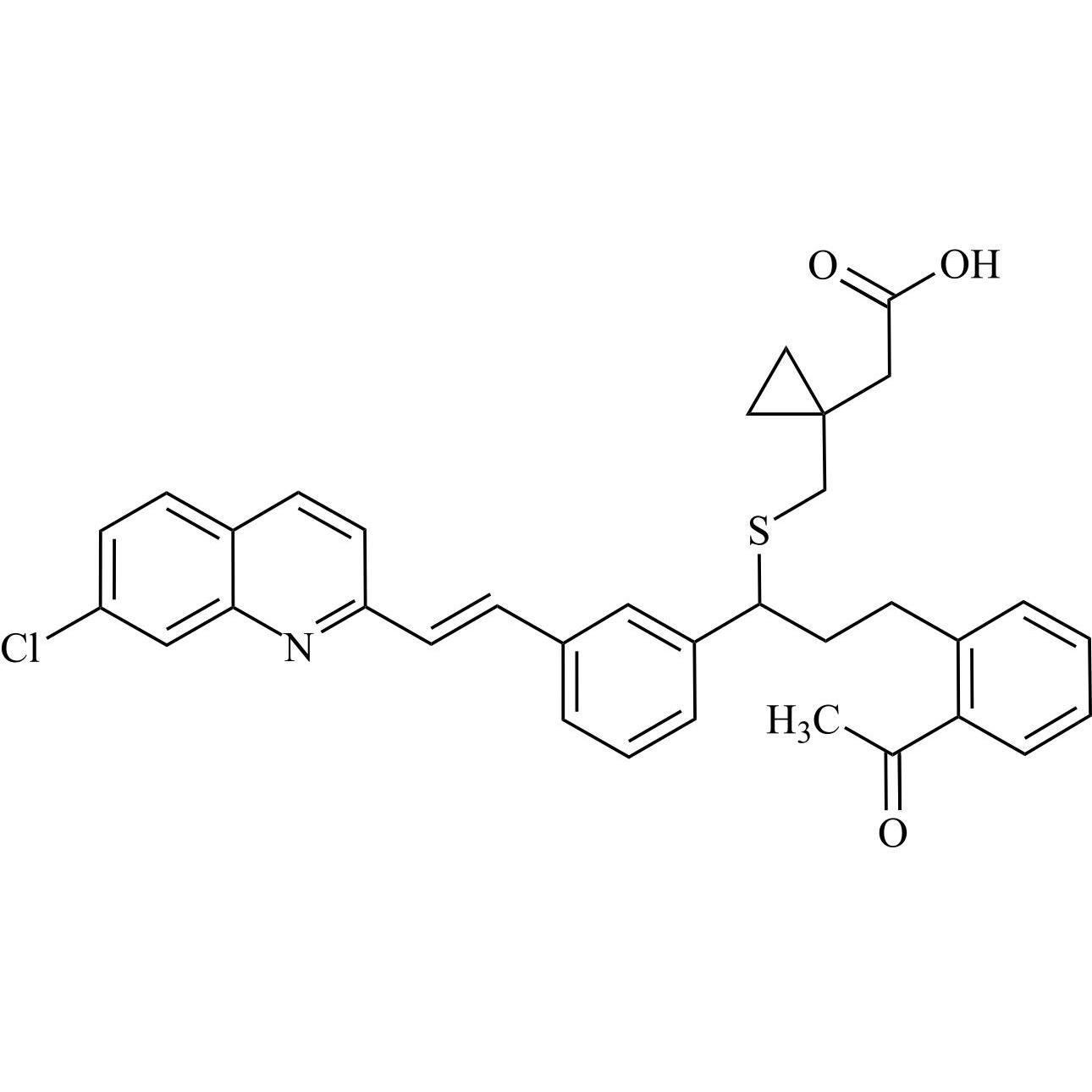
M.F.
M.W. 570.14
CAT# AR-M05076
CAS# NA
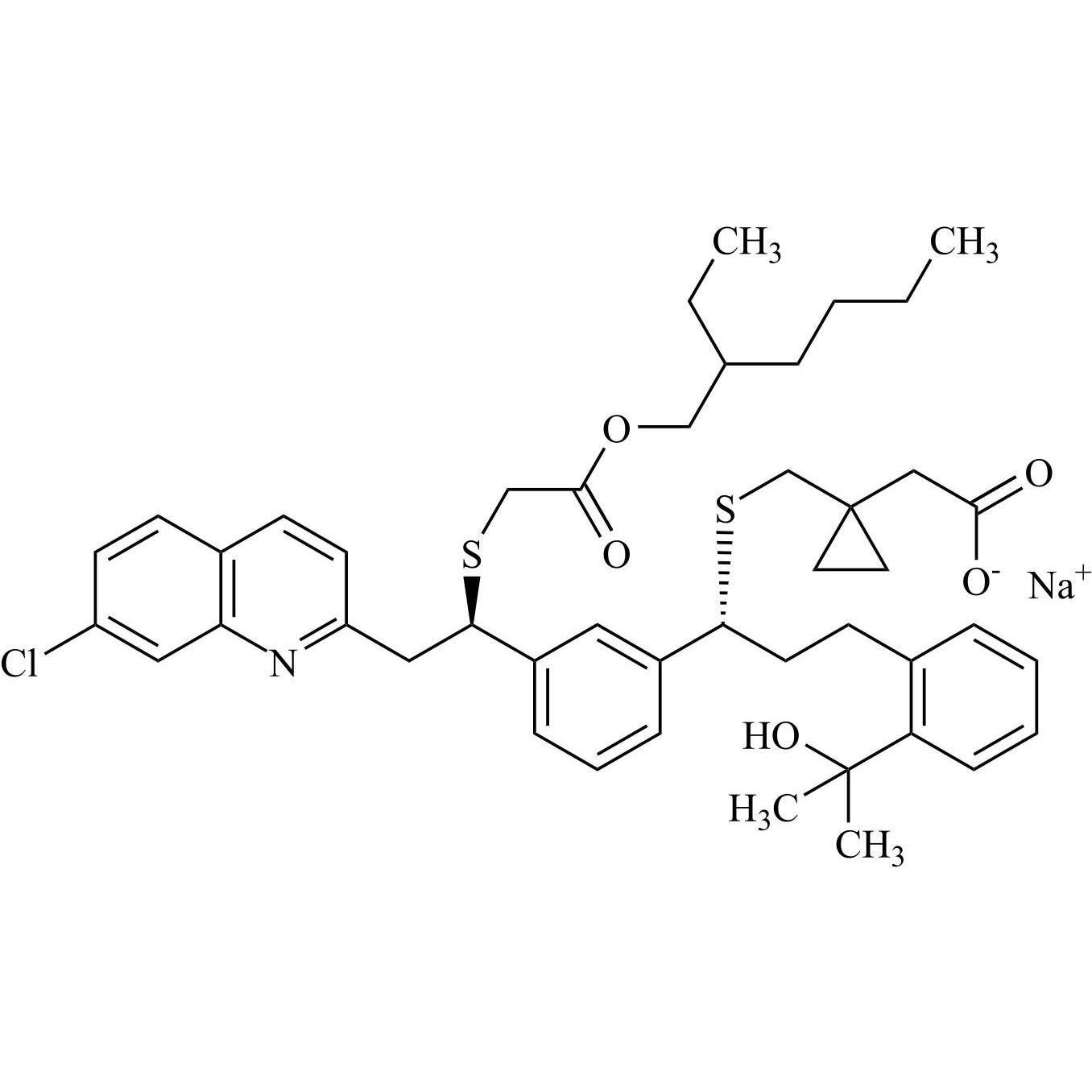
Montelukast Impurity 36 and Montelukast Impurity 37 (Mixture of Diastereomers)
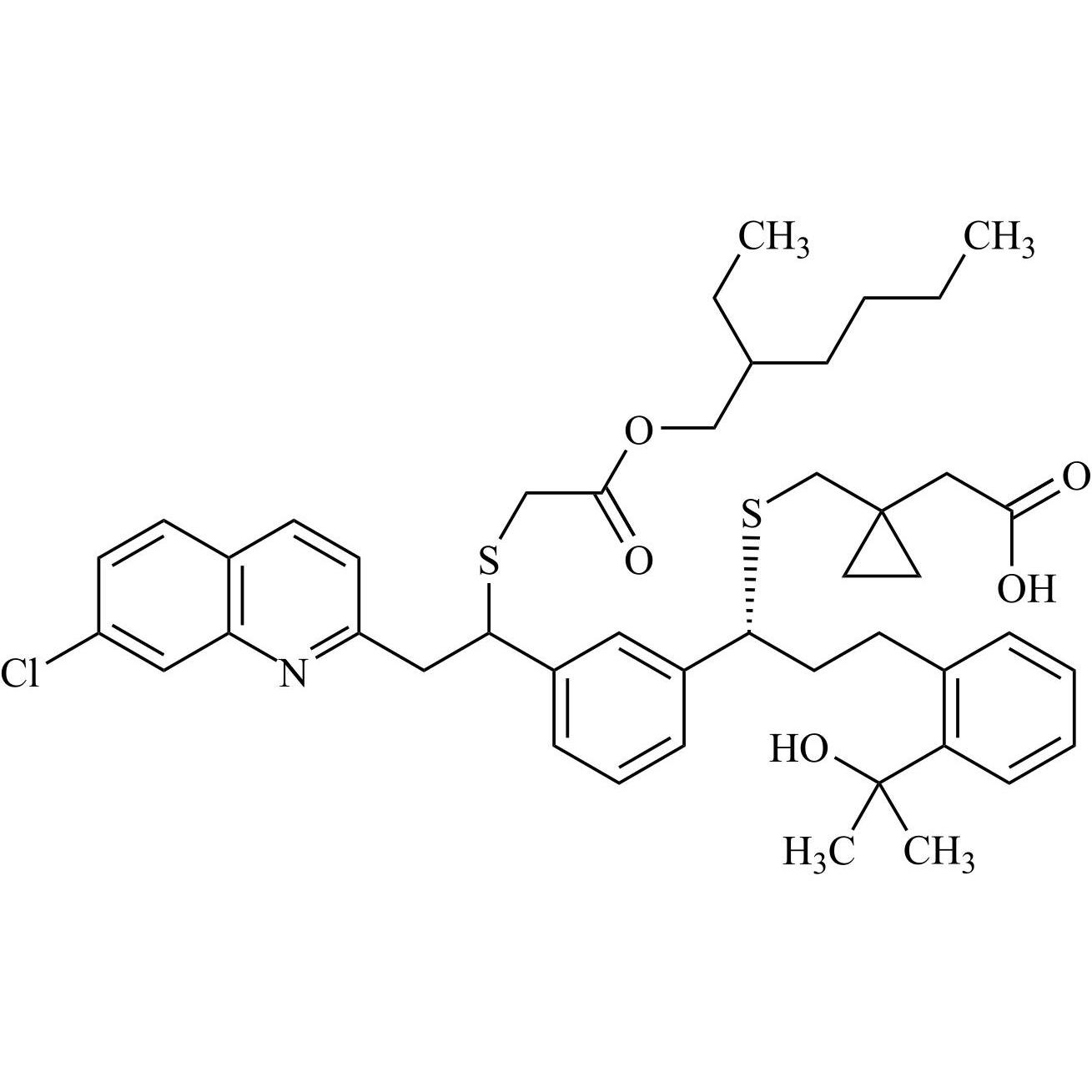
M.F.
M.W. 790.52
CAT# AR-M05078
CAS# NA