- Synonyms1H-2-Methylimidazole; 2-Methyl-1H-imidazole; 2MZ; 2MZ-H; 2MZ-PW; Actiron 2MI; Curezol 2MZ; Curezol 2MZ-P; Denka CN 25; Epicure MI 2; Epikure MI 2; Imicure AMI 2;
- Description
1H-2-Methylimidazole; 2-Methyl-1H-imidazole; 2MZ; 2MZ-H; 2MZ-PW; Actiron 2MI; Curezol 2MZ; Curezol 2MZ-P; Denka CN 25; Epicure MI 2; Epikure MI 2; Imicure AMI 2;
Ondansetron EP Impurity F is a fully characterized chemical compound used as a reference standard of API Ondansetron. The standard offered is compliant with regulatory guidelines. Ondansetron EP Impurity F is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 693-98-1
Related products
Ondansetron Hydrochloride hydrate

M.F.
M.W. 293.37; 36.46; 1.2(18.02)
CAT# AR-O08810
CAS# 103639-04-9
Ondansetron Related Compound C (Ondansetron EP Impurity C)

M.F.
M.W. 199.25
CAT# AR-O22916
CAS# 27387-31-1
Ondansetron Related Compound A (USP)-d6
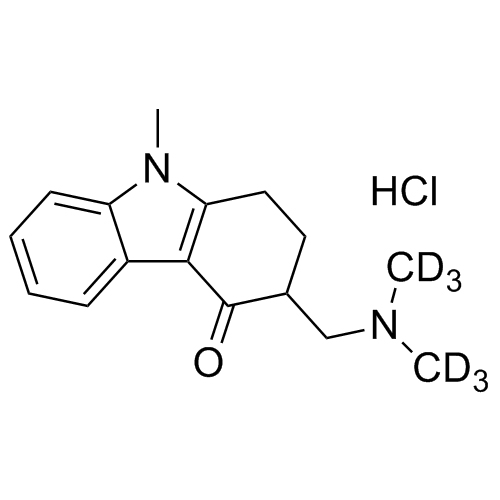
M.F.
M.W. 262.39 36.46
CAT# AR-O01396
CAS# 119812-29-2 (non-labelled)





















