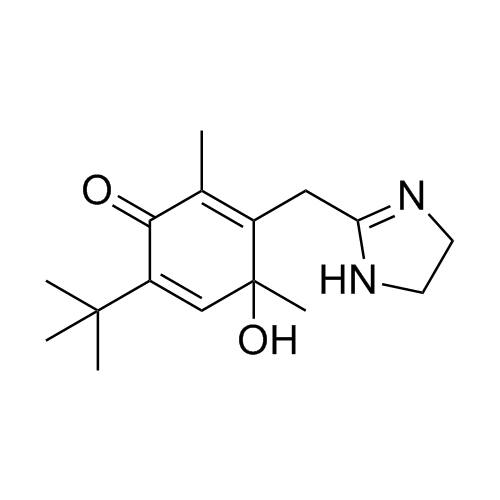
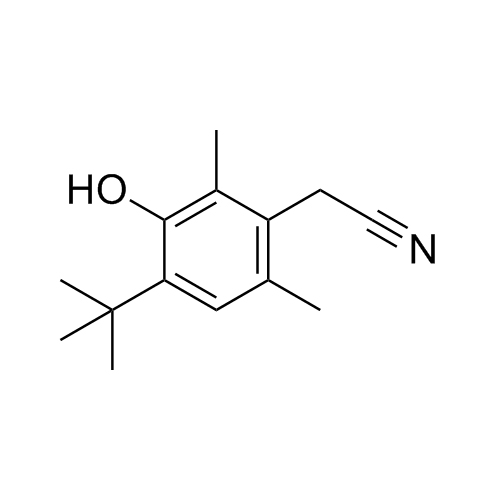
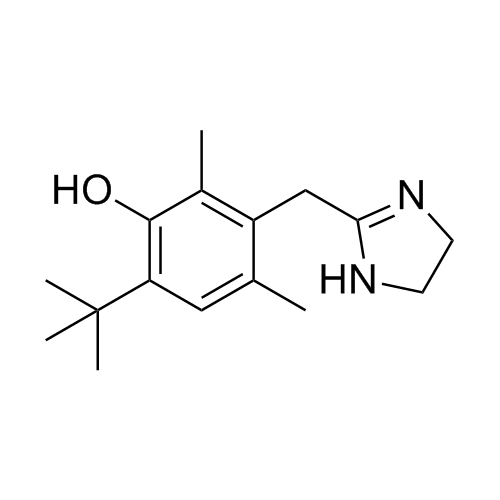
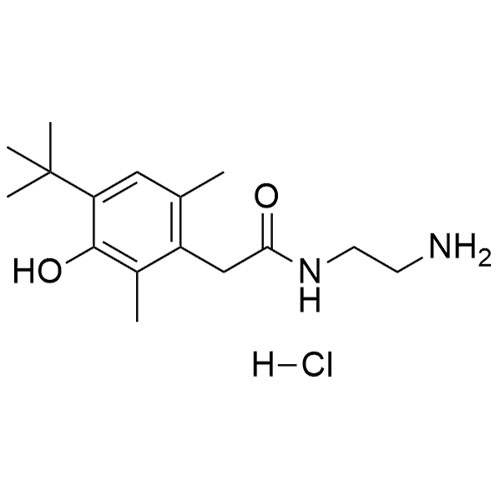
2-(4-(tert-butyl)-2,6-dimethylbenzyl)-4,5-dihydro-1H-imidazole hydrochloride;2-[[4-(1,1-Dimethylethyl)-2,6-dimethylphenyl]methyl]-4,5-dihydro-1H-imidazole Hydrochlroide; 2-(4-tert-Butyl-2,6-dimethylbenzyl)-2-imidazoline Monohydrochloride; Brizolin; Galazolin; Neo-Rinoleina; Neo-Synephrine II; Novorin; Olynth; Otrivin Hydrochloride; Therapin; Xymelin;Xylometazoline Hydrochloride; (Xylometazoline HCl)
Oxymetazoline EP Impurity B HCl is a fully characterized chemical compound used as a reference standard of API Oxymetazoline. The standard offered is compliant with regulatory guidelines. Oxymetazoline EP Impurity B HCl is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 1218-35-5