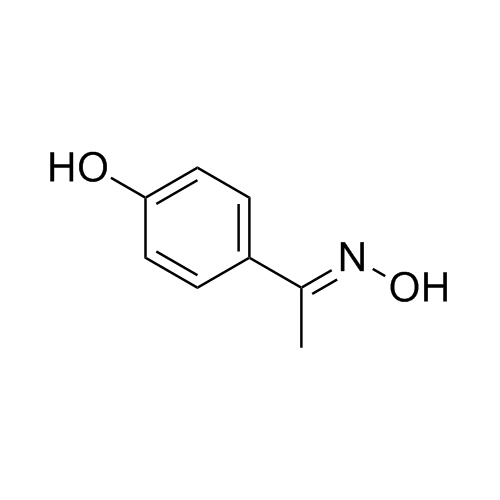
- Synonyms1-(4-hydroxyphenyl)ethanone;1-(4-Hydroxyphenyl)ethanone; 4-Acetophenol; 4-Acetylphenol; 4-Hydroxyphenyl Methyl Ketone; Methyl 4-Hydroxyphenyl Ketone; Piceol; p-Acetophenol; p-Acetylphenol; p-Hydoxyacetophenone; NSC 3698; Paracetamol EP Impurity E
- Description
1-(4-hydroxyphenyl)ethanone;1-(4-Hydroxyphenyl)ethanone; 4-Acetophenol; 4-Acetylphenol; 4-Hydroxyphenyl Methyl Ketone; Methyl 4-Hydroxyphenyl Ketone; Piceol; p-Acetophenol; p-Acetylphenol; p-Hydoxyacetophenone; NSC 3698; Paracetamol EP Impurity E
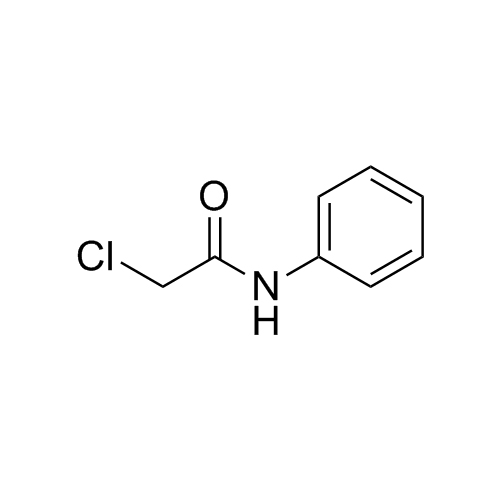
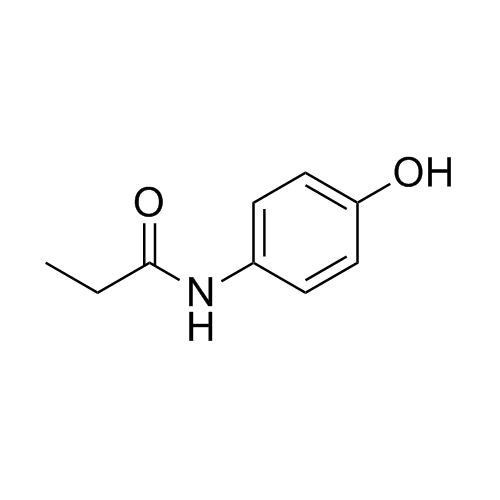
Paracetamol EP Impurity E is a fully characterized chemical compound used as a reference standard of API Acetaminophen. The standard offered is compliant with regulatory guidelines. Paracetamol EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 99-93-4
Related products
N-(5-acetamido-2-hydroxyphenyl)-N-(4-hydroxyphenyl)acetamide
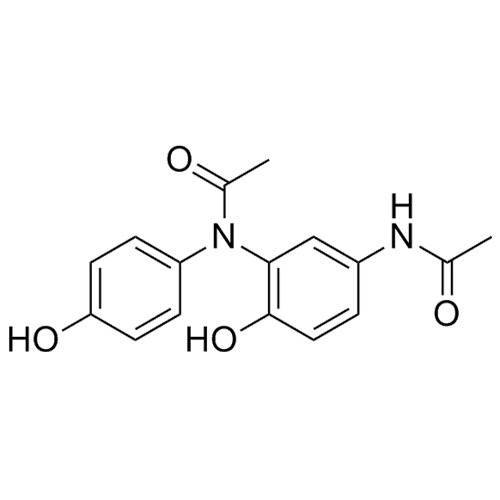
M.F.
M.W. 300.31
CAT# AR-A01190
CAS# 98966-17-7
Bis(p-acetylaminophenyl) Ether (Acetaminophen Impurity)
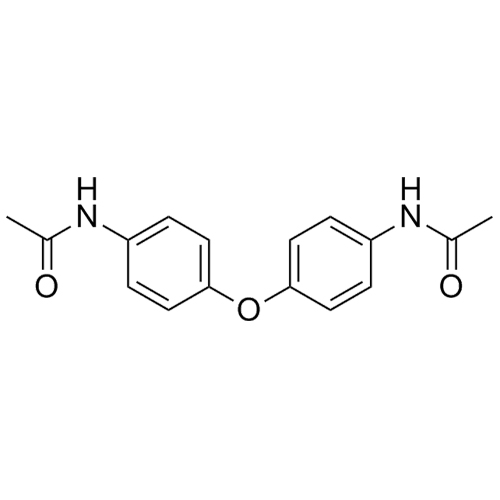
M.F.
M.W. 284.31
CAT# AR-A01186
CAS# 3070-86-8
Acetaminophen Related Compound C (Paracetamol EP Impurity A)
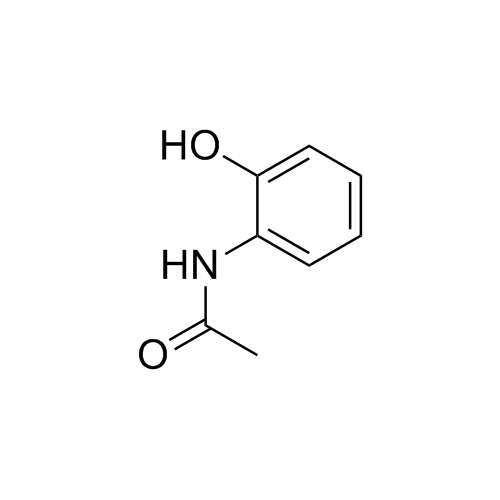
M.F.
M.W. 151.16
CAT# AR-A01410
CAS# 614-80-2
Acetaminophen Impurity K HCl (Paracetamol Impurity K HCl)

M.F.
M.W. 109.13; 36.46
CAT# AR-A01173
CAS# 51-78-5



![Show details for N,N'-(2-hydroxy-[1,1'-biphenyl]-4,4'-diyl)diacetamide Picture of N,N'-(2-hydroxy-[1,1'-biphenyl]-4,4'-diyl)diacetamide](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-A01184.jpg?size=256)