- SynonymsN-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-D-glutamic Acid; (2R)-2-[[4-(2-[2-Amino-4-oxo-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-5-yl]ethyl)phenyl]formamido]pentanedioic Acid
- Description
N-[4-[2-(2-Amino-4,7-dihydro-4-oxo-3H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-D-glutamic Acid; (2R)-2-[[4-(2-[2-Amino-4-oxo-3H,4H,7H-pyrrolo[2,3-d]pyrimidin-5-yl]ethyl)phenyl]formamido]pentanedioic Acid
Pemetrexed EP Impurity E is a fully characterized chemical compound used as a reference standard of API Pemetrexed. The standard offered is compliant with regulatory guidelines. Pemetrexed EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 182009-04-7
Related products
Pemetrexed Impurity 7 (Mixture of Diastereomers)
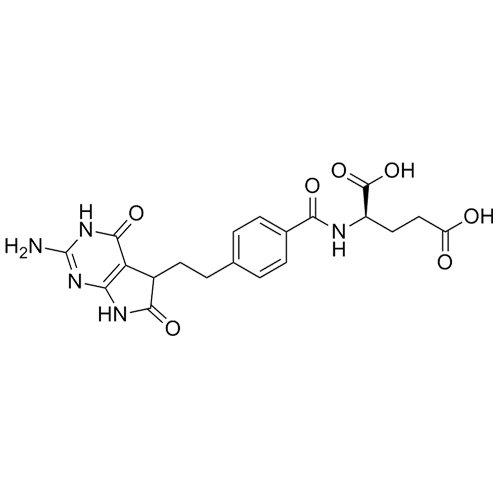
M.F.
M.W. 443.42
CAT# AR-P01341
CAS# 1644287-27-3 & 193281-00-4
Pemetrexed Disodium Heptahydrate

M.F.
M.W. 427.4 : 2(23.0) : 7(18.0)
CAT# AR-P29572
CAS# 357166-29-1
Pemetrexed EP Impurity B and Pemetrexed EP Impurity C
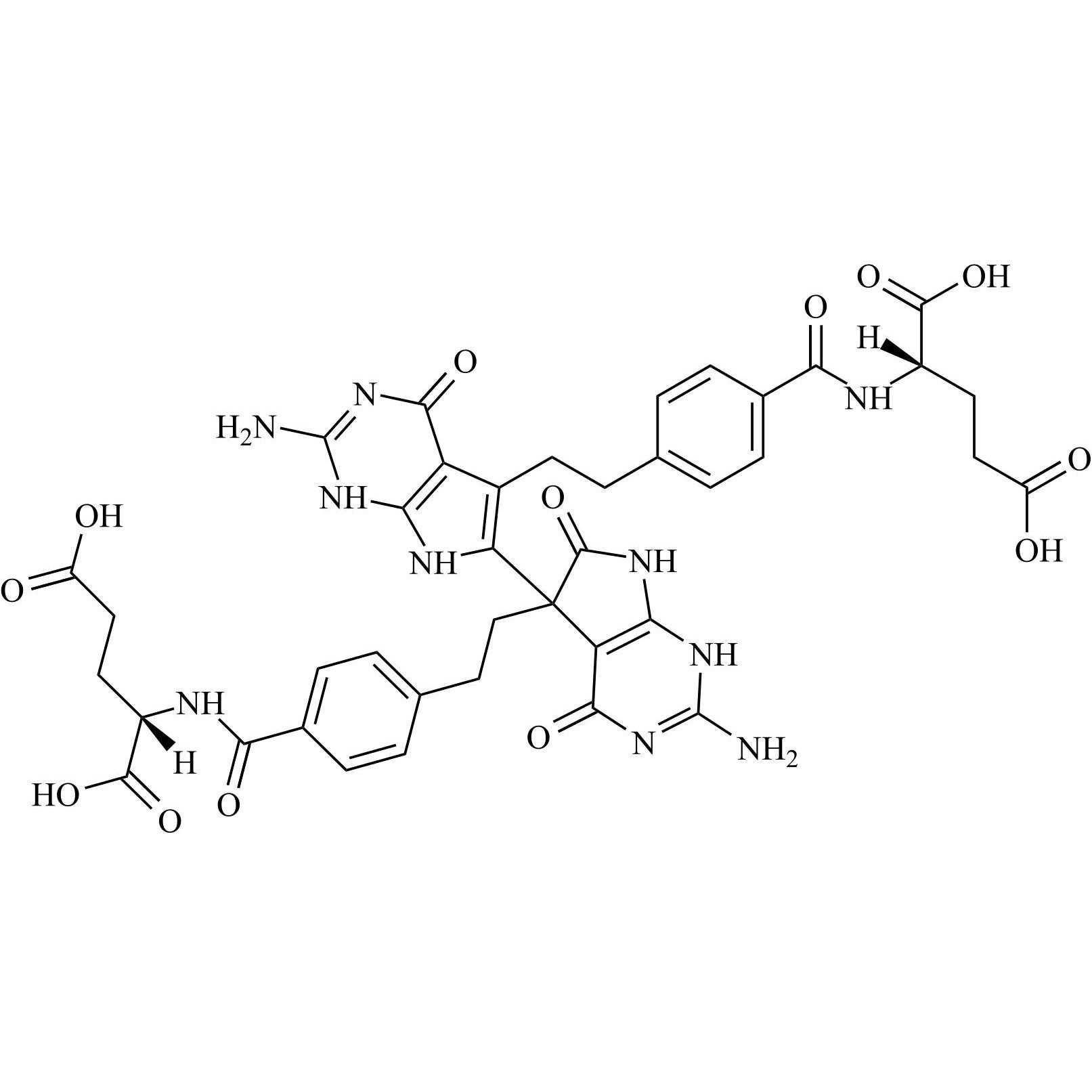
M.F.
M.W. 868.82
CAT# AR-P30581
CAS# 1644286-34-9
Pemetrexed-13C5 Disodium Salt
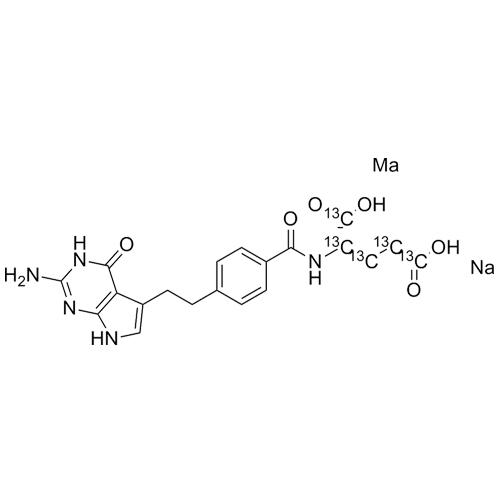
M.F.
M.W. 430.35 2 22.99
CAT# AR-P01332
CAS# 150399-23-8 (non-labelled)












































































