- Synonyms(8S,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-10,13-dimethyl-7,8,9,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthrene-3,11(6H)-dione
- Description
(8S,9S,10R,13S,14S,17R)-17-acetyl-17-hydroxy-10,13-dimethyl-7,8,9,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthrene-3,11(6H)-dione
Prednisone Impurity 8 is a fully characterized chemical compound used as a reference standard of API Prednisone. The standard offered is compliant with regulatory guidelines. Prednisone Impurity 8 is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS -
Related products
9b,11b-Epoxy-17,21-dihydroxypregna-1,4-diene-3,20-dione
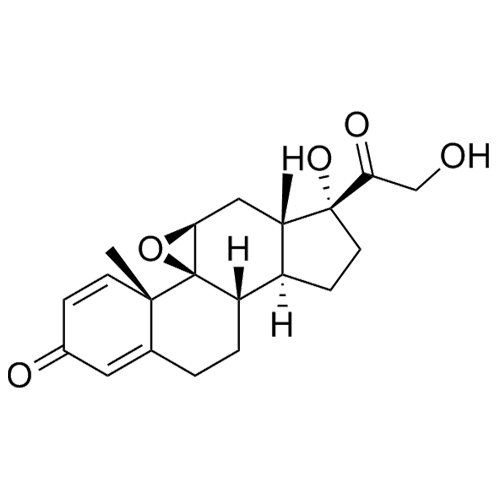
M.F.
M.W. 358.44
CAT# AR-P02715
CAS# 7091-05-6
17-Hydroxy-3,11-dioxo-androsta-1,4-diene-17?-carboxylic Acid
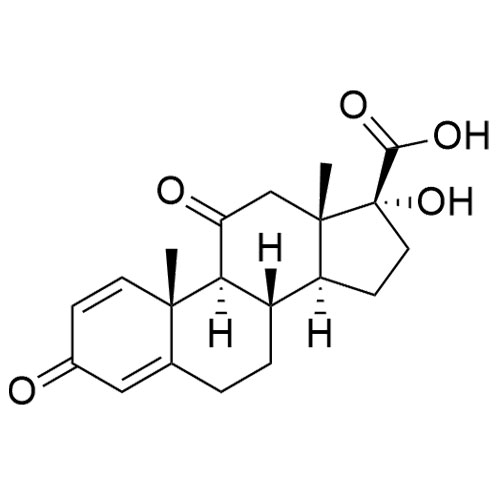
M.F.
M.W. 344.4
CAT# AR-P29390
CAS# 78261-67-3
Prednisone Impurity 29 (20(R)-Hydroxy Methylprednisolone)
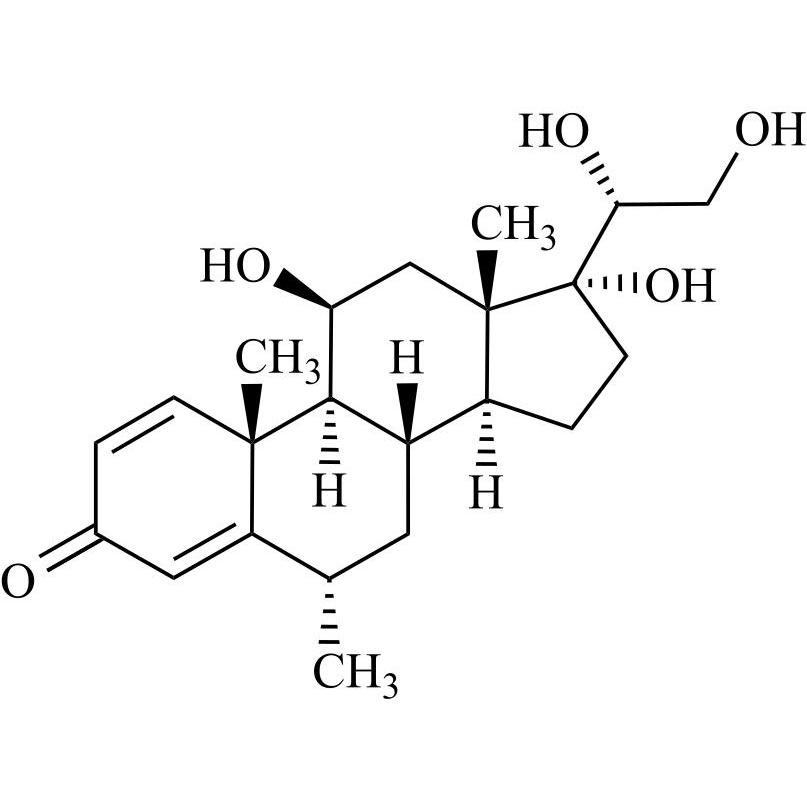
M.F.
M.W. 376.49
CAT# AR-P32486
CAS# 387-65-5
Prednisone Impurity 30
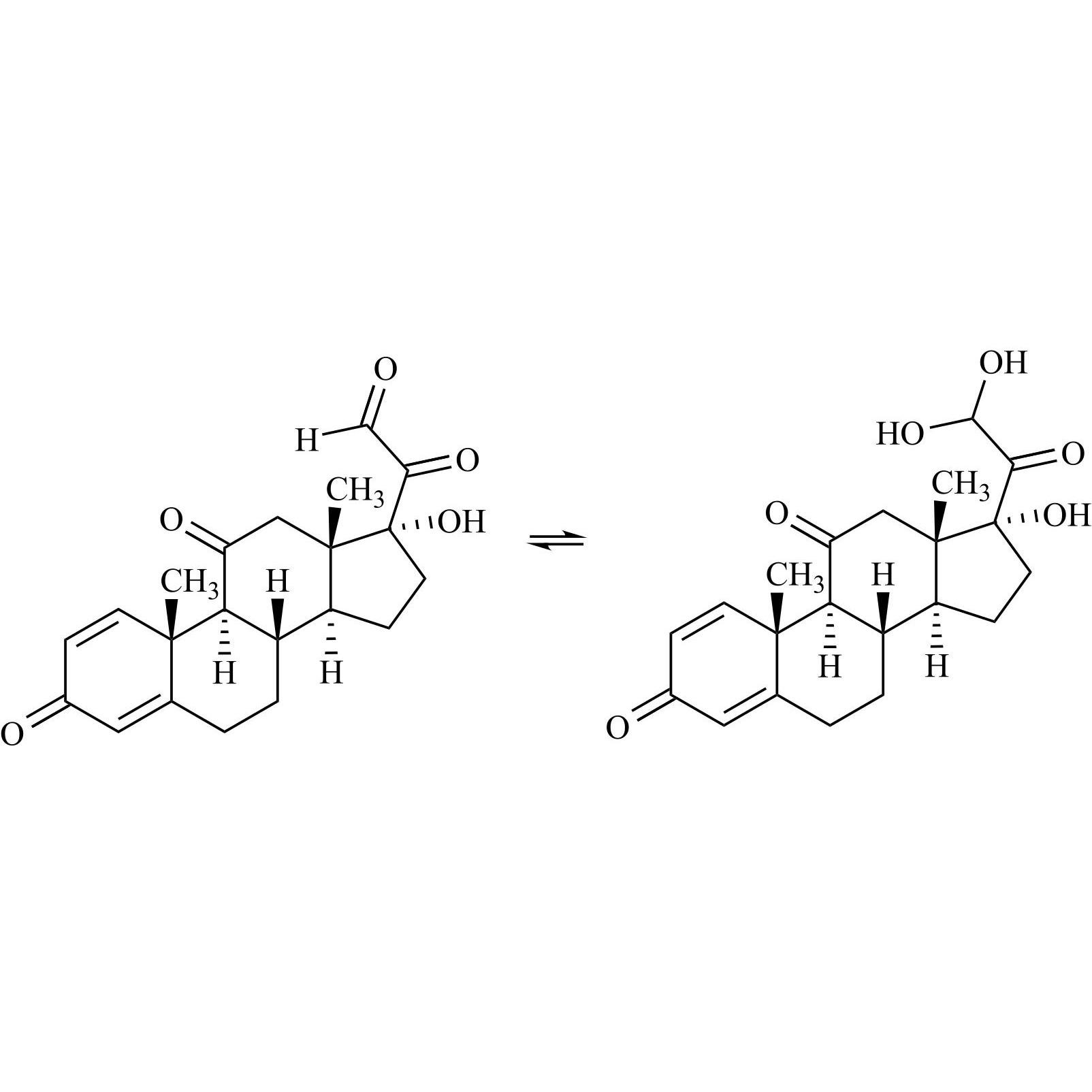
M.F.
M.W. 356.42 374.43
CAT# AR-P32489
CAS# 70522-55-3 (aldehyde form), 500198-20-9 (diol form)




















































