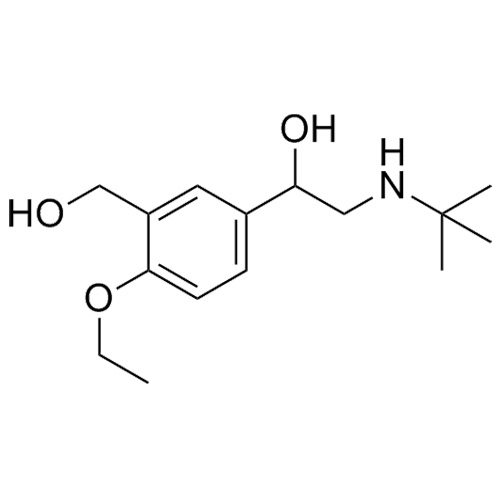
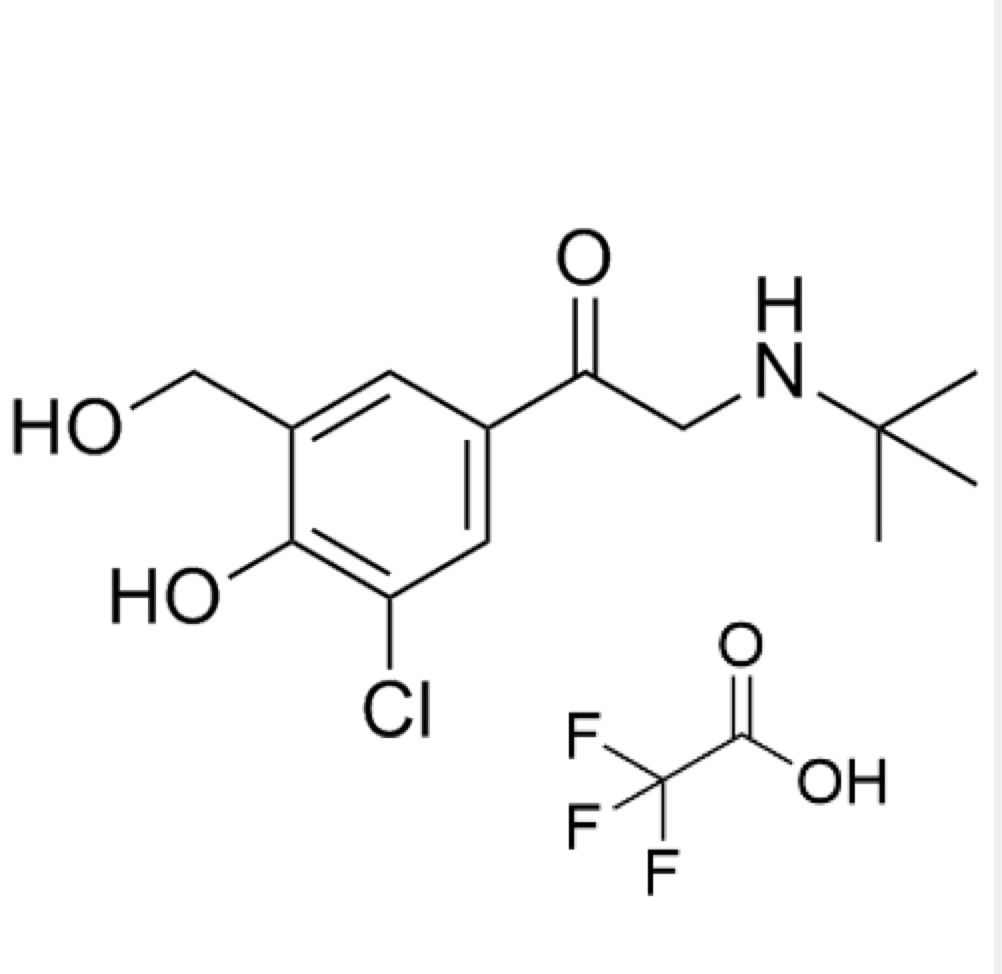
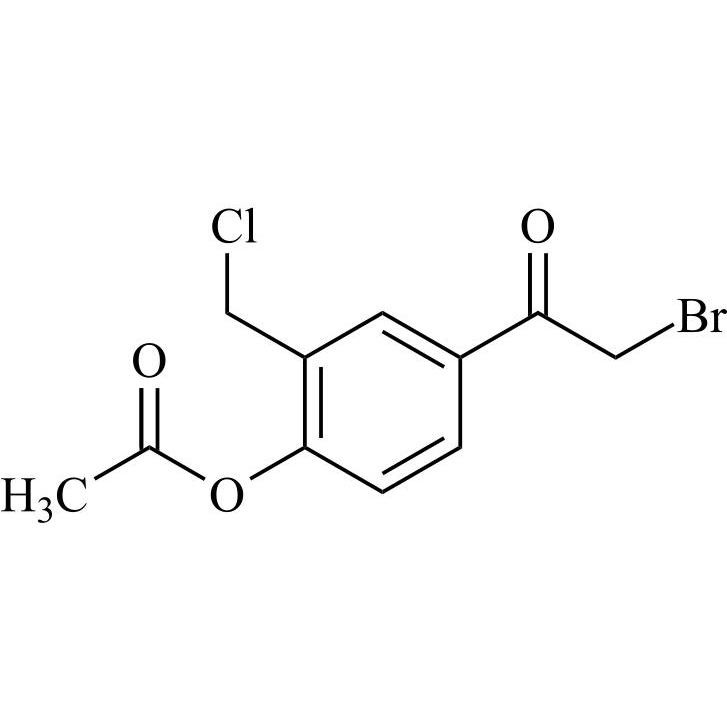
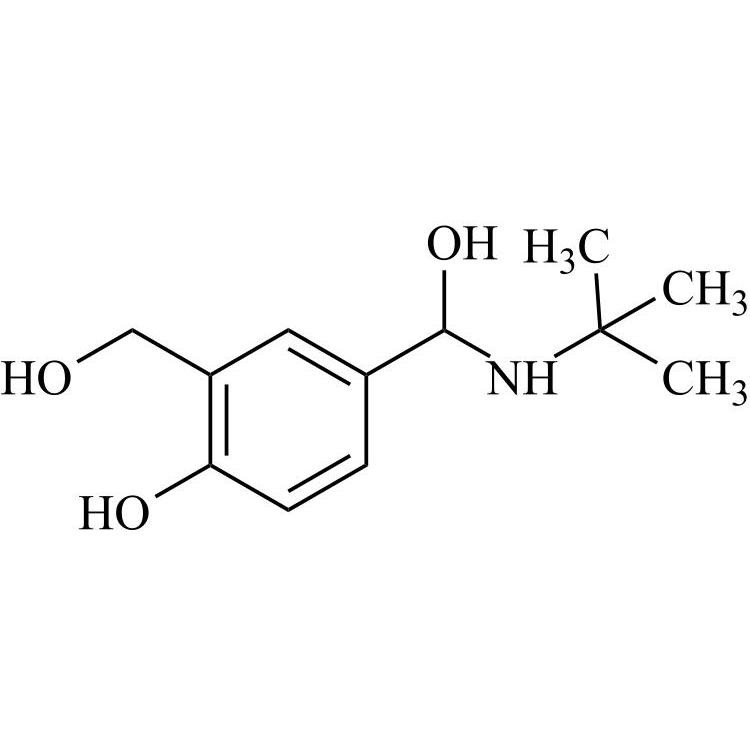
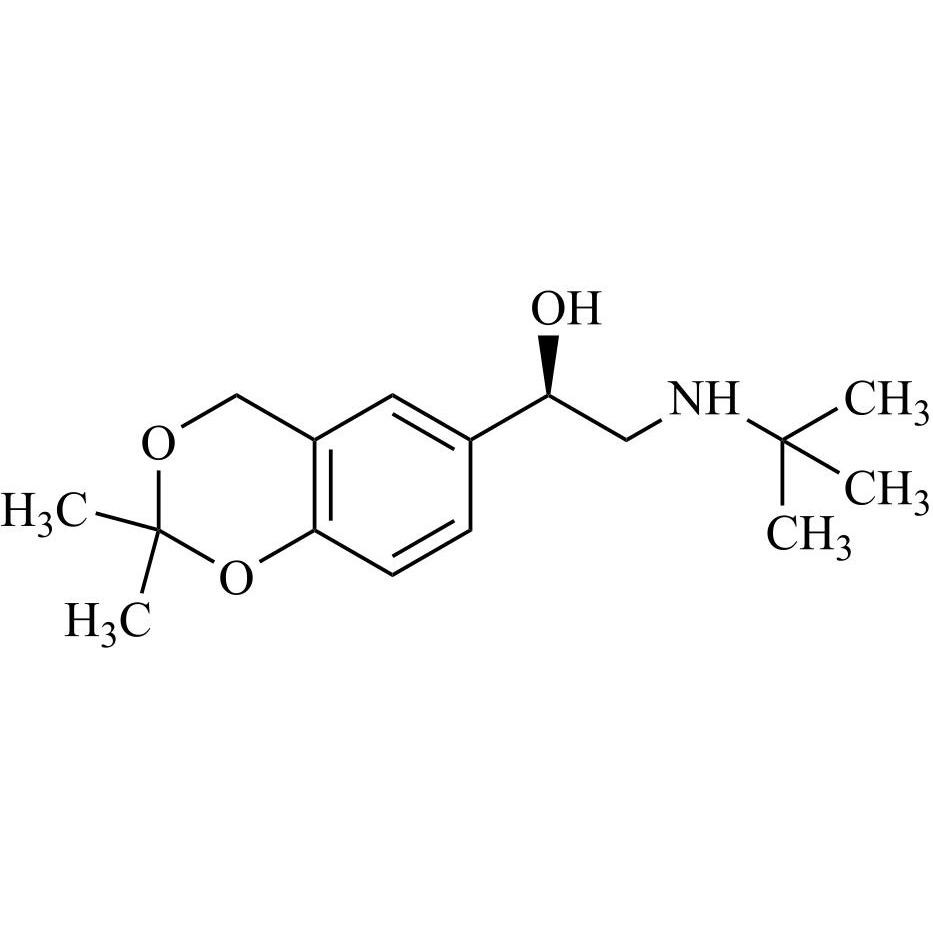
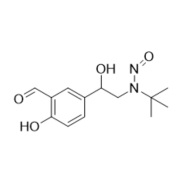
- Synonyms4-(2-(benzyl(tert-butyl)amino)-1-hydroxyethyl)-2-(hydroxymethyl)phenol;?1-[[(1,1-Dimethylethyl)(phenylmethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol; ?1-[(Benzyl-tert-butylamino)methyl]-4-hydroxy-m-xylene-?,?’-diol; ?1-Benzyl-tert-butylaminomethyl-4-hydroxy-m-xylene-?1,?3-diol; 1-(4-Hydroxy...
- Description
4-(2-(benzyl(tert-butyl)amino)-1-hydroxyethyl)-2-(hydroxymethyl)phenol;?1-[[(1,1-Dimethylethyl)(phenylmethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol; ?1-[(Benzyl-tert-butylamino)methyl]-4-hydroxy-m-xylene-?,?’-diol; ?1-Benzyl-tert-butylaminomethyl-4-hydroxy-m-xylene-?1,?3-diol; 1-(4-Hydroxy-3-hydroxymethylphenyl)-2-(tert-butylbenzylamino)ethanol; Salbutamol EP Impurity E;N-Benzyl Albuterol
Salbutamol EP Impurity E is a fully characterized chemical compound used as a reference standard of API Salbutamol. The standard offered is compliant with regulatory guidelines. Salbutamol EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 24085-03-8
Related products
Salbutamol EP Impurity N Diacetate (Mixture of Diastereomers)

M.F.
M.W. 460.62 2*60.05
CAT# AR-S09844
CAS# NA
Salbutamol EP Impurity D Hemisulfate (Levalbuterol USP Related Compound D)

M.F.
M.W. 237.30 0.5*98.07
CAT# AR-S09848
CAS# NA