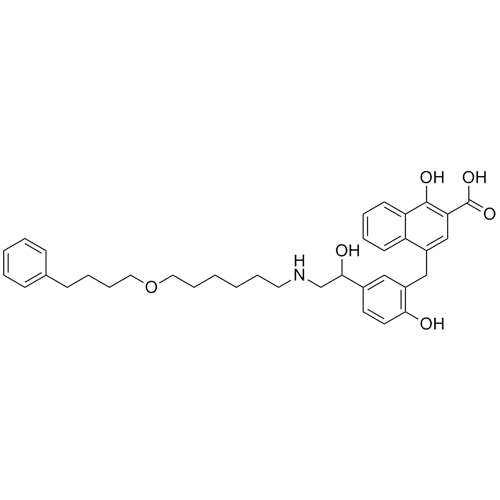
- Synonyms4-(1-hydroxy-2-((6-((4-phenylbutan-2-yl)oxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenol;4-Hydroxy-?1-[[[6-(1-methyl-3-phenylpropoxy)hexyl]amino]methyl]-1,3-benzenedimethanol (Salmeterol Impurity);USP Salmeterol Related Compound B; Salmeterol Impurity E
- Description
4-(1-hydroxy-2-((6-((4-phenylbutan-2-yl)oxy)hexyl)amino)ethyl)-2-(hydroxymethyl)phenol;4-Hydroxy-?1-[[[6-(1-methyl-3-phenylpropoxy)hexyl]amino]methyl]-1,3-benzenedimethanol (Salmeterol Impurity);USP Salmeterol Related Compound B; Salmeterol Impurity E
Salmeterol EP Impurity E is a fully characterized chemical compound used as a reference standard of API Salmeterol. The standard offered is compliant with regulatory guidelines. Salmeterol EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 108928-81-0
Related products
(R)-methyl 2-(benzyloxy)-5-(2-bromo-1-hydroxyethyl)benzoate
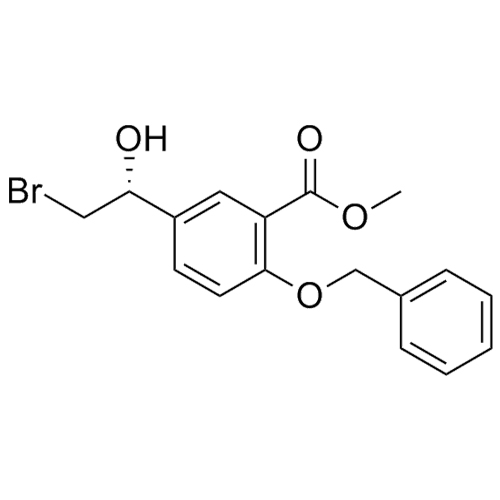
M.F.
M.W. 365.23
CAT# AR-S01118
CAS# 160889-18-9