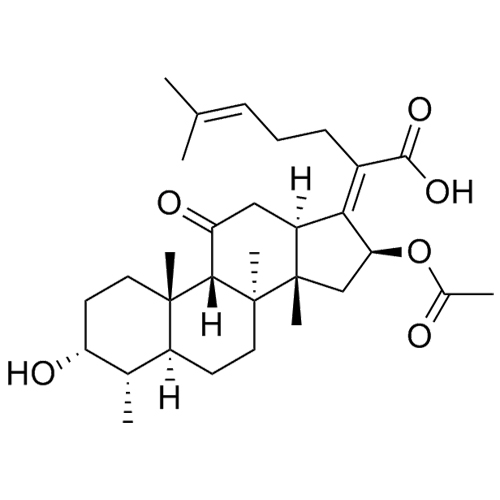
- Synonyms(3?,4?,8?,9?,11?,13?,14?,16?,17Z)-3,11,16-Trihydroxy-29-nordammara-17(20),24-dien-21-oic Acid;Deacetyl-16-epifusidic Acid; (Z)-3?,11?,16?-trihydroxy-29-Nor-8?,9?,13?,14?-dammara-17(20),24-dien-21-oic Acid; 16-Epideacetylfusidic acid; NSC 319631; Fusidic Acid EP Impurity I;16-Epi-deacetyl-fusidic...
- Description
(3?,4?,8?,9?,11?,13?,14?,16?,17Z)-3,11,16-Trihydroxy-29-nordammara-17(20),24-dien-21-oic Acid;Deacetyl-16-epifusidic Acid; (Z)-3?,11?,16?-trihydroxy-29-Nor-8?,9?,13?,14?-dammara-17(20),24-dien-21-oic Acid; 16-Epideacetylfusidic acid; NSC 319631; Fusidic Acid EP Impurity I;16-Epi-deacetyl-fusidic Acid
Sodium Fusidate EP Impurity I is a fully characterized chemical compound used as a reference standard of API Fusidic Acid. The standard offered is compliant with regulatory guidelines. Sodium Fusidate EP Impurity I is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 5951-83-7
Related products
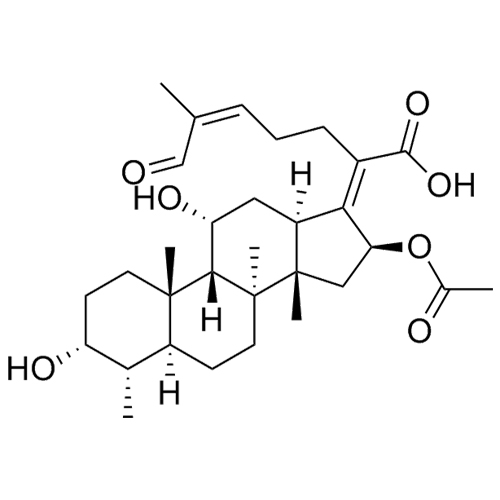
Sodium Fusidate EP Impurity A (Mixture of Diastereomers)

M.F.
M.W. 550.73
CAT# AR-F02237
CAS# 80445-74-5