- Synonyms7-[(3-Chloro-6-methyldibenzo[c,f][1,2]thiazepin-11(6H)-ylidene)amino]-heptanoic Acid S,S-dioxide; Dibenzo[c,f][1,2]thiazepine Heptanoic Acid Deriv; Tianeptine Impurity D
- Description
7-[(3-Chloro-6-methyldibenzo[c,f][1,2]thiazepin-11(6H)-ylidene)amino]-heptanoic Acid S,S-dioxide; Dibenzo[c,f][1,2]thiazepine Heptanoic Acid Deriv; Tianeptine Impurity D
Tianeptine EP Impurity D is a fully characterized chemical compound used as a reference standard of API Tianeptine. The standard offered is compliant with regulatory guidelines. Tianeptine EP Impurity D is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 131206-48-9
Related products
Tianeptine EP Impurity B Fumarate

M.F.
M.W. 465.02 116.07
CAT# AR-T02037
CAS# 66981-77-9 (free base)
Tianeptine MC5-d4 (pentanoic acid metabolite)
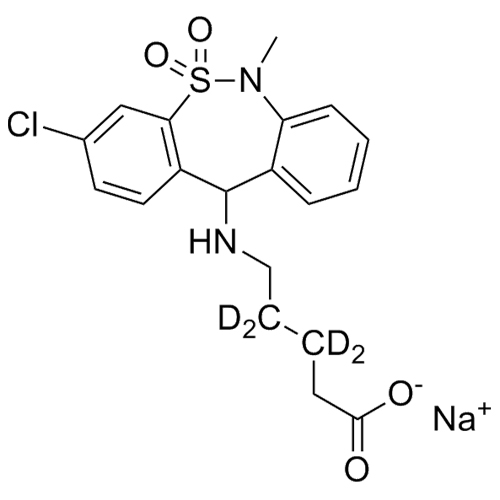
M.F.
M.W. 411.90 22.99
CAT# AR-T02034
CAS# 115220-11-6 (non-d)














