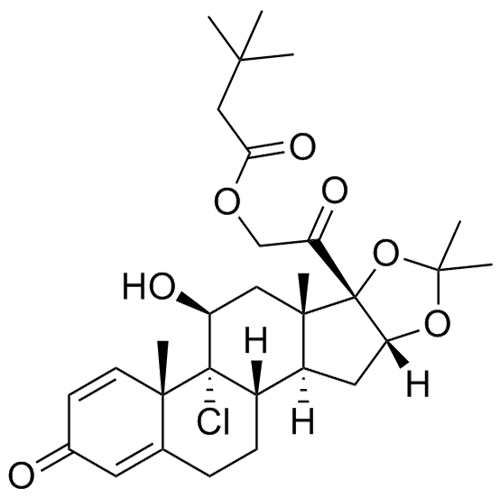
- Synonyms(6aS,6bR,7S,8aS,8bS,11aR,12aS,12bS)-6b-fluoro-7-hydroxy-8b-(2-hydroxyacetyl)-6a,8a,10,10-tetramethyl-5,6,6a,6b,7,8,8a,8b,11a,12,12a,12b-dodecahydro-1H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-4(2H)-one; 9-Fluoro-16?,17-(isopropylidenedioxy) Corticosterone; 11?,16?,17?,21-Tetrahydroxy-9?-fluorop...
- Description
(6aS,6bR,7S,8aS,8bS,11aR,12aS,12bS)-6b-fluoro-7-hydroxy-8b-(2-hydroxyacetyl)-6a,8a,10,10-tetramethyl-5,6,6a,6b,7,8,8a,8b,11a,12,12a,12b-dodecahydro-1H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-4(2H)-one; 9-Fluoro-16?,17-(isopropylidenedioxy) Corticosterone; 11?,16?,17?,21-Tetrahydroxy-9?-fluoropregn-4-ene-3,20-dione 16,17-Acetonide; (11?,16?)-9-Fluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]pregn-4-ene-3,20-dione; 9-fluoro-11?,16?,17,21-Tetrahydroxypregn-4-ene-3,20-dione cyclic 16,17-Acetal with Acetone; NSC 21915; 9-Fluoro-16alpha,17-(isopropylidenedioxy) Corticosterone; (11β,16α)-9-fluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis(oxy)]-Pregn-4-ene-3,20-dione;
Triamcinolone Acetonide EP Impurity E is a fully characterized chemical compound used as a reference standard of API Triamcinolone. The standard offered is compliant with regulatory guidelines. Triamcinolone Acetonide EP Impurity E is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 1524-86-3
Related products
Triamcinolone Hexacetonide Impurity B (Mixture of Diastereomers)

M.F.
M.W. 518.62
CAT# AR-T02929
CAS# NA
1,2-dihydro Triamcinolone 16,17-Acetonide 21-mesylate

M.F.
M.W. 514.62
CAT# AR-T02924
CAS# 2367-73-9
Triamcinolone Acetonide EP Impurity F (Triamcinolone Acetonide 21-Acetate)

M.F.
M.W. 476.54
CAT# AR-T02909
CAS# 3870-07-3
21-Methoxy Triamcinolone Acetonide (Mixture of Diastereomers)

M.F.
M.W. 464.53
CAT# AR-T05843
CAS# 161740-70-1