rac-Phenylephrine EP Impurity D HCl is a fully characterized chemical compound used as a reference standard of API Lumacaftor. The standard offered is compliant with regulatory guidelines. rac-Phenylephrine EP Impurity D HCl is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA
Related products
Phenylephrine Impurity 19 (Mixture of Diastereomers)
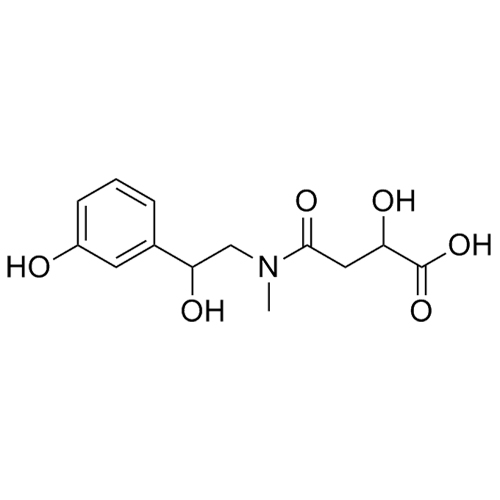
M.F.
M.W. 283.28
CAT# AR-P01721
CAS# 1217525-08-0
2-methyl-1,2,3,4-tetrahydroisoquinoline-4,8-diol

M.F.
M.W. 179.22 36.46
CAT# AR-P01732
CAS# 23824-25-1
2-amino-1-(3-hydroxyphenyl)ethanone hydrochloride

M.F.
M.W. 151.17 36.46
CAT# AR-P01746
CAS# 14665-75-9
N-Methyl-3,3-diphenylpropylamine Acetate Salt

M.F.
M.W. 225.34 60.05
CAT# AR-P01763
CAS# 28075-29-8 (free base)
(1S,4R)-1,2,3,4-Tetrahydro-2-methyl-1-phenyl-4,6-isoquinolinediol,

M.F. -
M.W. -
CAT# AR-P29689
CAS# 2095343-19-2
(1R,4R)-1,2,3,4-Tetrahydro-2-methyl-1-phenyl-4,6-isoquinolinediol
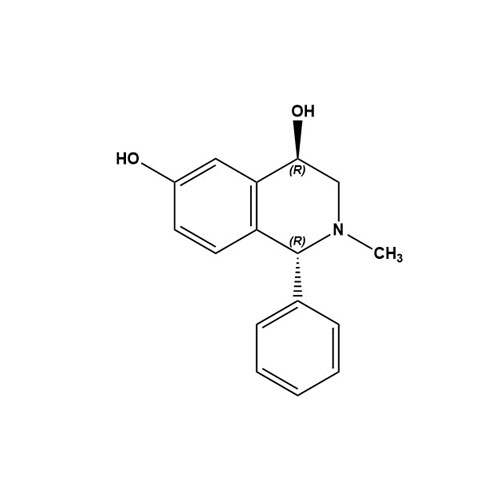
M.F. -
M.W. -
CAT# AR-P29690
CAS# 2095343-25-0
N-(2-Succinyl) Phenylephrine (Mixture of Diastereomers)

M.F.
M.W. 283.28
CAT# AR-P01734
CAS# 915278-80-7
Phenylephrine Related Compound F HCl

M.F.
M.W. 179.22; 36.46; 18.02
CAT# AR-P29642
CAS# 1007885-60-0



























































