- Synonyms((2R,3R,4S,5R)-3-(Benzoyloxy)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-4-methyltetrahydrofuran-2-yl)methyl benzoate
- Description
((2R,3R,4S,5R)-3-(Benzoyloxy)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-hydroxy-4-methyltetrahydrofuran-2-yl)methyl benzoate
Sofosbuvir Impurity 8 is a fully characterized chemical compound used as a reference standard of API Sofosbuvir. The standard offered is compliant with regulatory guidelines. Sofosbuvir Impurity 8 is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 1910099-11-4
Related products
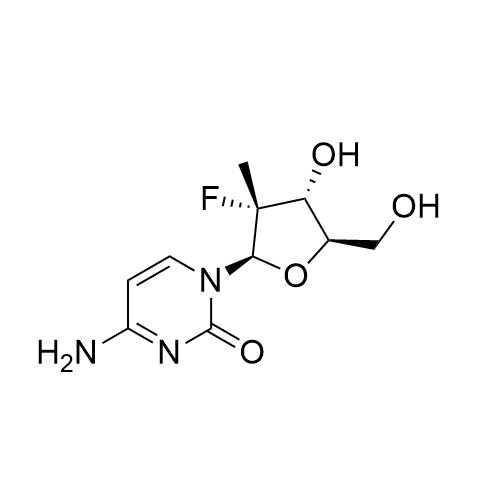
(2'R)-2'-Deoxy-2'-fluoro-2'-methyl-uridine 5'-Triphosphate

M.F.
M.W. 500.16
CAT# AR-S01678
CAS# 1015073-42-3
Sofosbuvir (2’R)-2’-Chloro-2’-deoxy-2’-methyl-uridine

M.F.
M.W. 276.68
CAT# AR-S01682
CAS# 1496551-72-4
(2R)-2-Deoxy-2-fluoro-2-methyl-D-erythropentonic Acid γ-Lactone 3,5-Dibenzoate

M.F.
M.W. 372.35
CAT# AR-S01677
CAS# 874638-80-9
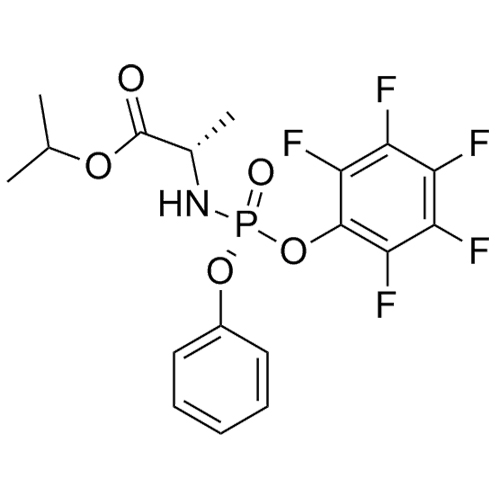
Sofosbuvir (R)-Phosphate (RP-Isomer Of Sofosbuvir)

M.F.
M.W. 529.46
CAT# AR-S01665
CAS# 1190308-01-0
Sofosbuvir Impurity 49 (GS-566500) Ditriethylamine Salt

M.F.
M.W. 411.28 2*101.19
CAT# AR-S10615
CAS# NA
Sofosbuvir Impurity 34 Disodium Salt (GS-606965 Disodium Salt)

M.F.
M.W. 384.16 2*22.99
CAT# AR-S10616
CAS# NA
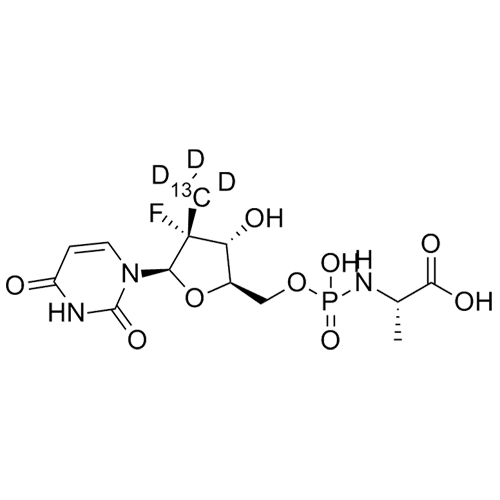
Sofosbuvir Impurity 49 (GS-566500)-13C-d3 Ditriethylamine Salt

M.F.
M.W. 415.29 2*101.19
CAT# AR-S10612
CAS# NA