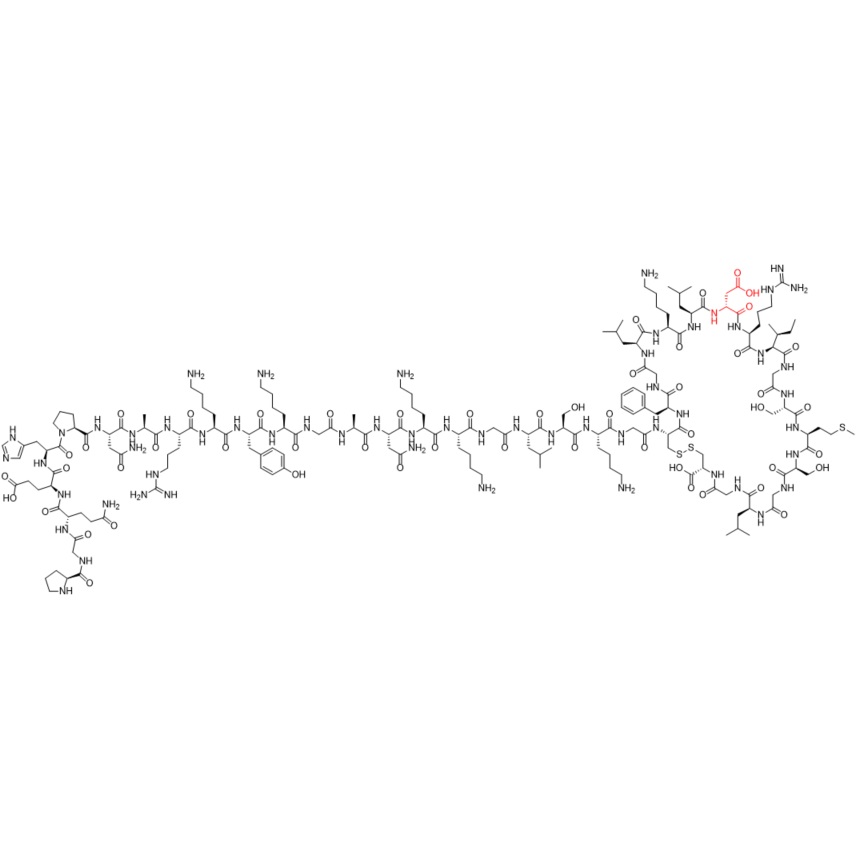
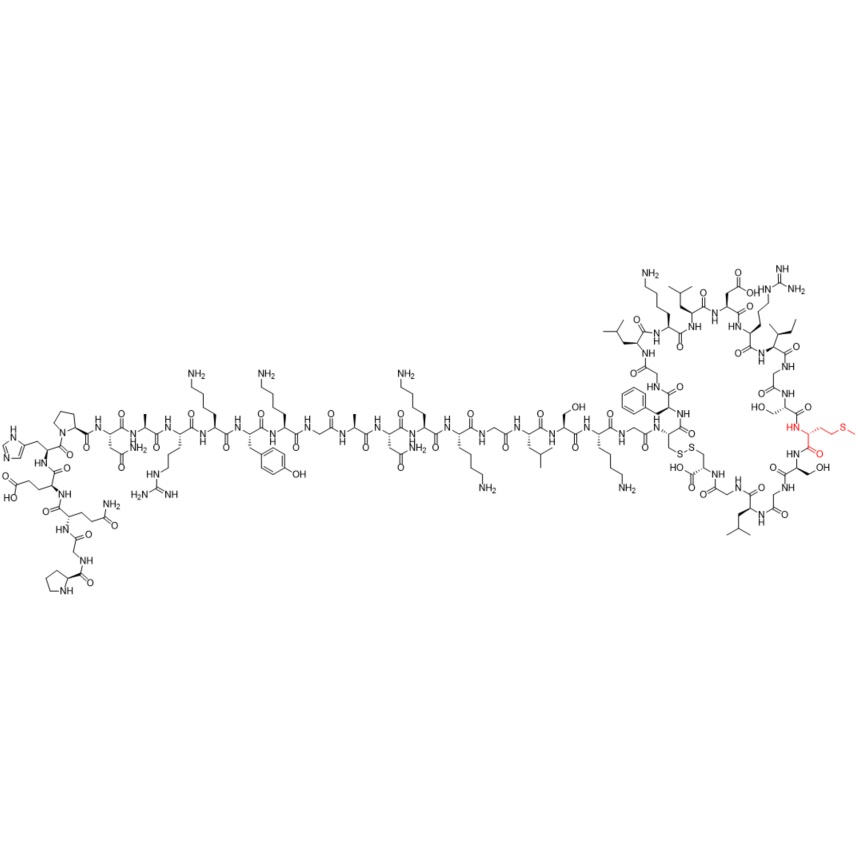
- SynonymsH-Pro-Gly-Gln-Glu-His-Pro-Asn-Ala-Arg-Lys-D-Tyr-Lys-Gly-Ala-Asn-Lys-Lys-Gly-Leu-Ser-Lys-Gly-Cys(1)-Phe-Gly-Leu-Lys-Leu-Asp-Arg-Ile-Gly-Ser-Met-Ser-Gly-Leu-Gly-Cys(1)-OH
- Description
H-Pro-Gly-Gln-Glu-His-Pro-Asn-Ala-Arg-Lys-D-Tyr-Lys-Gly-Ala-Asn-Lys-Lys-Gly-Leu-Ser-Lys-Gly-Cys(1)-Phe-Gly-Leu-Lys-Leu-Asp-Arg-Ile-Gly-Ser-Met-Ser-Gly-Leu-Gly-Cys(1)-OH
Vosoritide D-Tyr-11 Impurity is a fully characterized chemical compound used as a reference standard of API Vosoritide. The standard offered is compliant with regulatory guidelines. Vosoritide D-Tyr-11 Impurity is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA
Related products
Vosoritide Impurity 1 Trifluoroacetate(Des Asp 15)

M.F.
M.W. 3988.67 3*114.02
CAT# AR-V07076
CAS# NA
Vosoritide Impurity 7 Trifluoroacetate (Acetylated Lysin 12)

M.F.
M.W. 4144.81 3*114.02
CAT# AR-V07082
CAS# NA
Vosoritide Impurity 8 Trifluoroacetate (Acetylated Lysin 16)

M.F.
M.W. 4144.81 3*114.02
CAT# AR-V07083
CAS# NA
Vosoritide Impurity 9 Trifluoroacetate (Acetylated Lysin 17)

M.F.
M.W. 4144.81 3*114.02
CAT# AR-V07084
CAS# NA
Vosoritide Impurity 10 Trifluoroacetate (Acetylated Lysin 21)

M.F.
M.W. 4144.81 3*114.02
CAT# AR-V07085
CAS# NA
Vosoritide Impurity 11 Trifluoroacetate (Acetylated Lysin 27)

M.F.
M.W. 4144.81 3*114.02
CAT# AR-V07086
CAS# NA