Erythromycin Related Products
Axios Research stands at the forefront of pharmaceutical reference standards ingenuity, providing high quality Erythromycin Reference Standards. These include both pharmacopeial and non-pharmacopeial Erythromycin impurities, metabolites, stable isotope products, and nitrosamines. Our Erythromycin impurity reference standards are essential for pharmaceutical research, aiding in product development, ANDA and DMF submissions, quality control (QC), method validation, and stability studies. They are also used in identifying unknown impurities and evaluating genotoxic potential. Our Erythromycin related products are meticulously characterized and come with a comprehensive Certificates of Analysis (COA) and analytical data that comply with regulatory standards.Erythromycin Impurity J (Roxithromycin EP Impurity C)
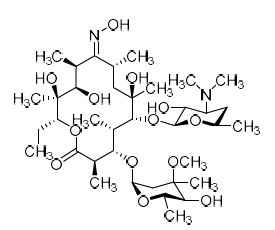
M.F.
M.W. 748.95
CAT# AR-E01801
CAS# 111321-02-9
Erythromycin EP Impurity E (Erythromycin A Enol Ether)
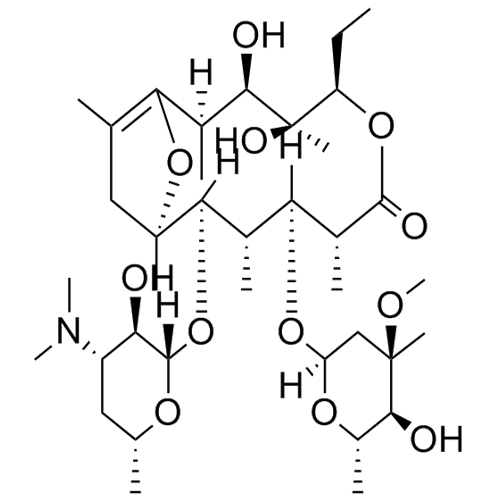
M.F.
M.W. 715.92
CAT# AR-E01814
CAS# 33396-29-1
2’,4’’-O-Bis(trimethylsilyl)-6-O-methylerythromycin A 9-[O-(1- methoxy-1-methylethyl) Oxime

M.F.
M.W. 979.45
CAT# AR-E01831
CAS# 119699-81-9










































