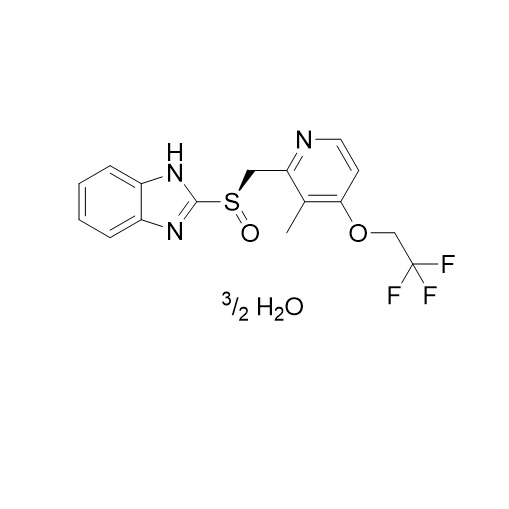
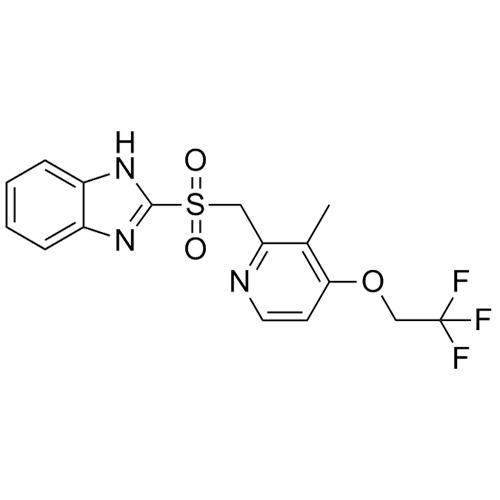
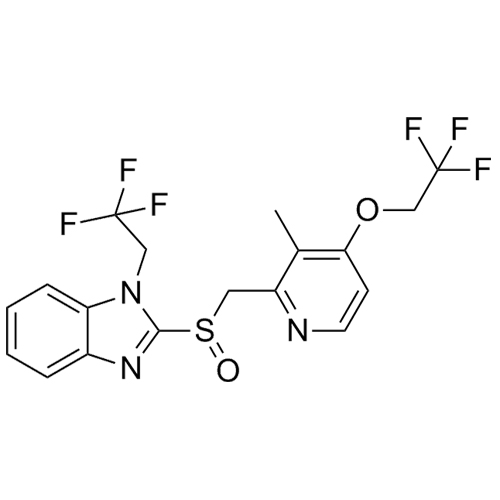
- Synonyms2-(((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)thio)-1H-benzo[d]imidazole; 2-[[[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]thio]-1H-benzimidazole; AG 1777; H 225/18; K 1252; Lansoprazole EP Impurity C; USP Impurity B
- Description
2-(((3-methyl-4-(2,2,2-trifluoroethoxy)pyridin-2-yl)methyl)thio)-1H-benzo[d]imidazole; 2-[[[3-Methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl]methyl]thio]-1H-benzimidazole; AG 1777; H 225/18; K 1252; Lansoprazole EP Impurity C; USP Impurity B
Lansoprazole EP Impurity C (USP Related Compound B) is a fully characterized chemical compound used as a reference standard of API Lansoprazole. The standard offered is compliant with regulatory guidelines. Lansoprazole EP Impurity C (USP Related Compound B) is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 103577-40-8
Related products
Lansoprazole EP Impurity C (USP Related Compound B)

M.F.
M.W. 353.36
CAT# AR-L01139
CAS# 103577-40-8
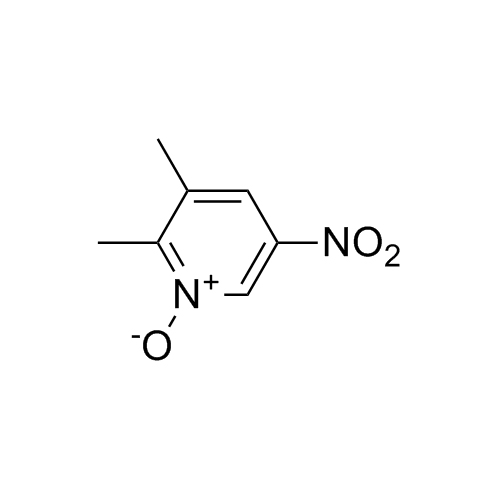
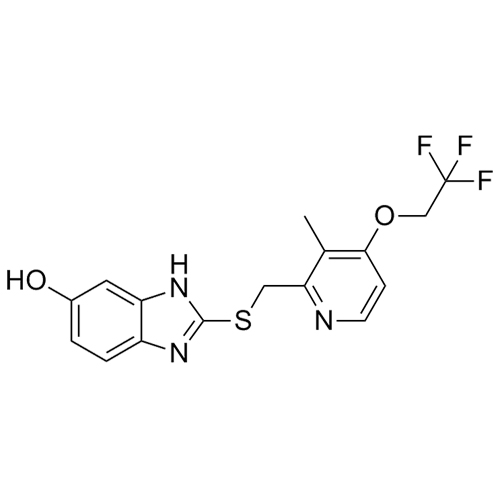
Rabeprazole EP Impurity K (Lansoprazole EP Impurity D)

M.F.
M.W. 134.14
CAT# AR-L01140
CAS# 615-16-7
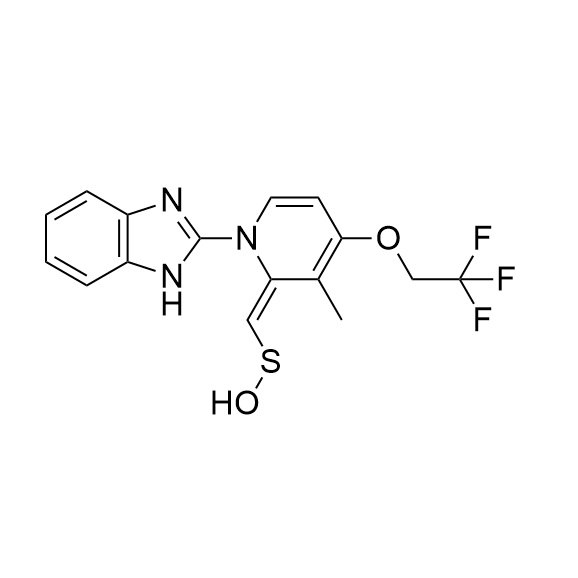
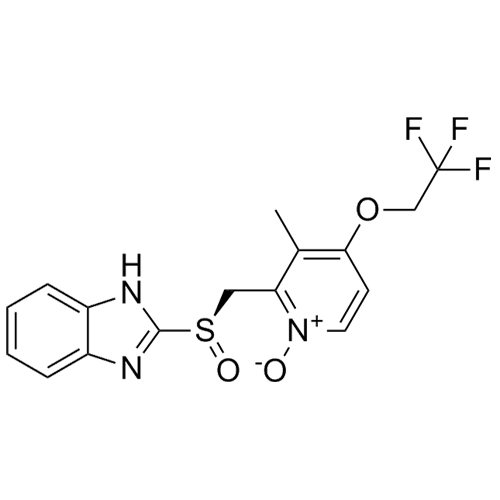
Lansoprazole N-(3-Methyl-4-Trifluoroethoxyl-Pyridin-2-yl) Impurity

M.F.
M.W. 572.53
CAT# AR-L01148
CAS# 1083100-26-8












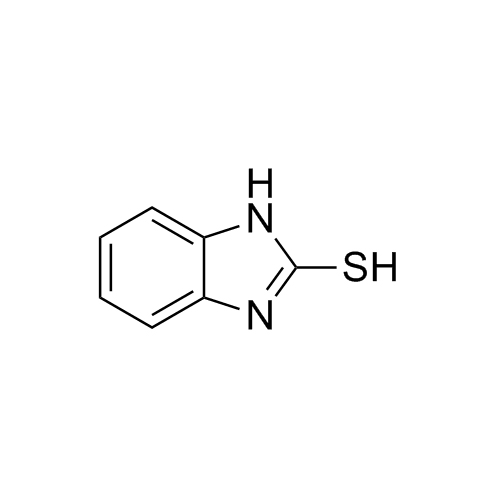



















![Show details for 2-mercapto-1H-benzo[d]imidazol-5-ol Picture of 2-mercapto-1H-benzo[d]imidazol-5-ol](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-L01168.jpg?size=256)