(2RS,3aRS,7aRS)-, (2′SR)-, (1″RS)-: (±)-2′-epi-perindopril (as per EP)
Perindopril EP Impurity P is a fully characterized chemical compound used as a reference standard of API Perindopril. The standard offered is compliant with regulatory guidelines. Perindopril EP Impurity P is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 82978-68-5
Related products
Perindopril Related Compound 4 HCl
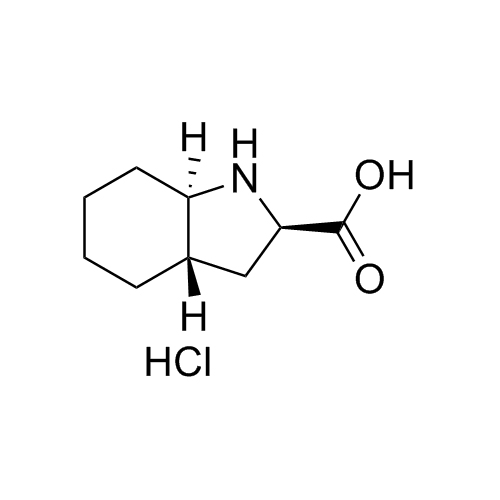
M.F.
M.W. 169.23 36.46
CAT# AR-P01491
CAS# 145513-92-4 (free base)
Perindopril Related Compound 5 HCl
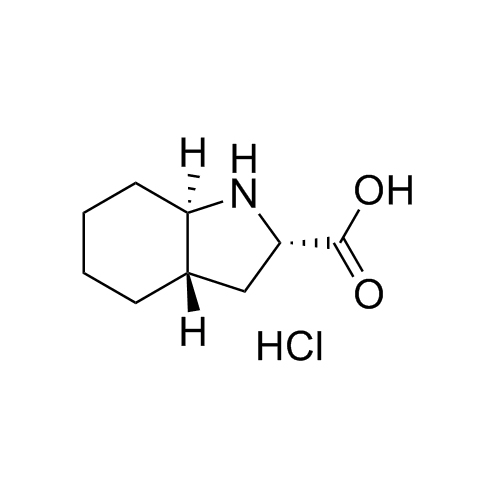
M.F.
M.W. 169.23 36.46
CAT# AR-P01492
CAS# 145513-93-5 (free base)
Perindopril Related Compound 5 Enantiomer HCl
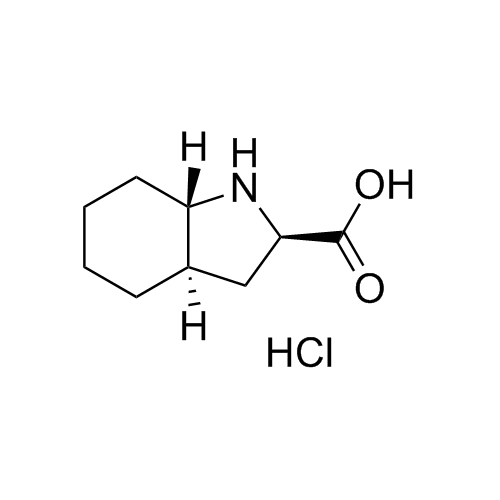
M.F.
M.W. 169.23 36.46
CAT# AR-P01500
CAS# 144540-74-9
Perindopril EP Impurity H (Mixture of Diastereomers)

M.F.
M.W. 528.74
CAT# AR-P29695
CAS# 353777-64-7
Perindopril EP Impurity I tert-Butylamine Salt
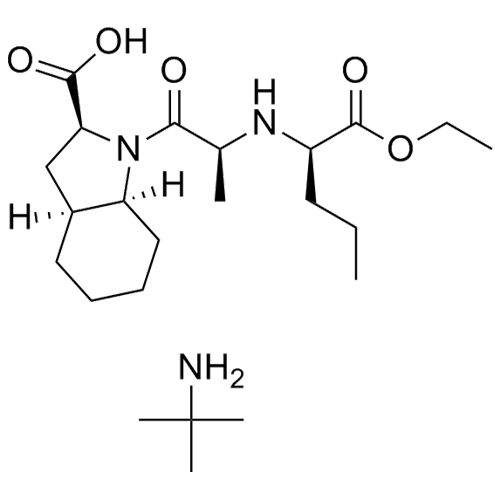
M.F.
M.W. 368.48 73.14
CAT# AR-P01481
CAS# 3081732-63-7
Perindopril EP Impurity F-d4 (Perindopril USP Related Compound F-d4)

M.F.
M.W. 354.48
CAT# AR-P30986
CAS# NA






















































































