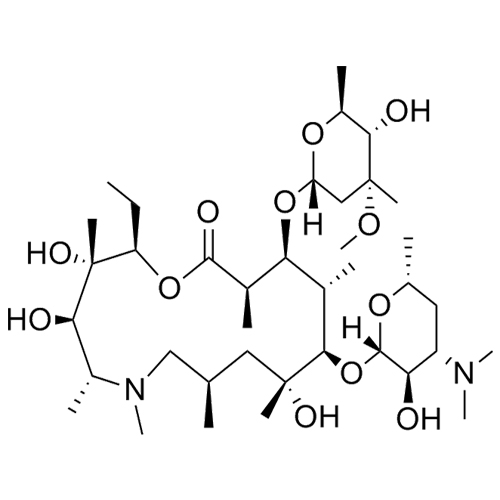
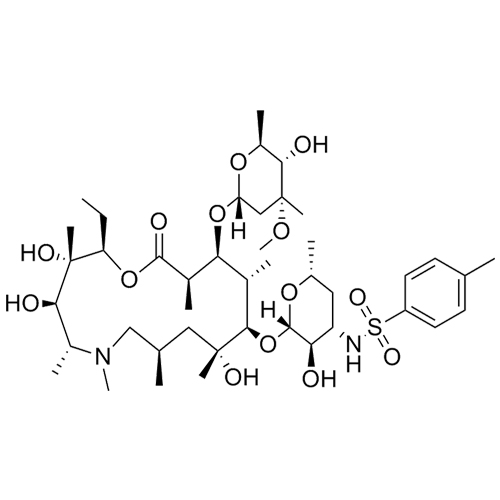
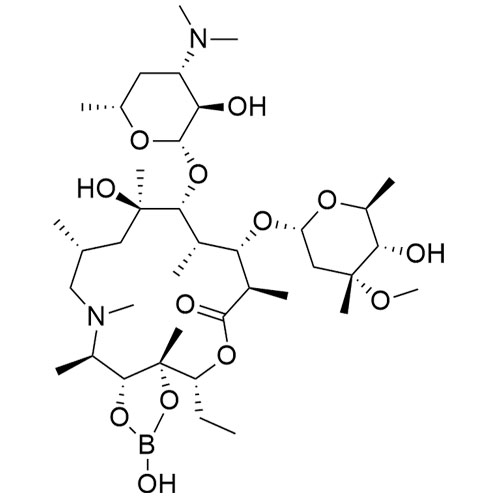
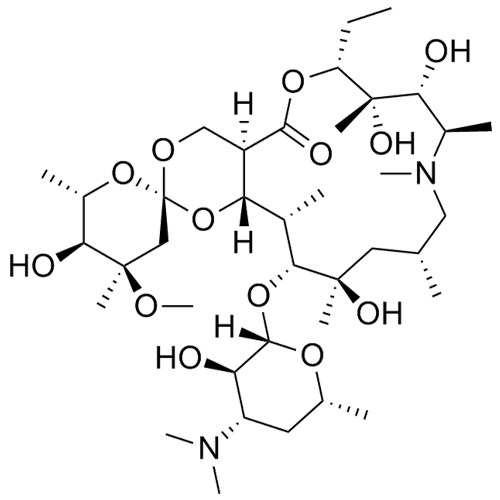
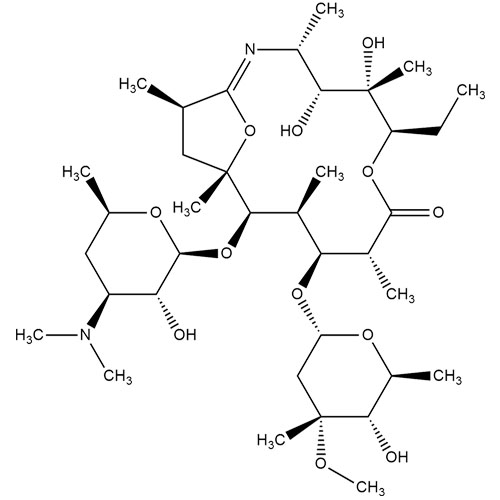
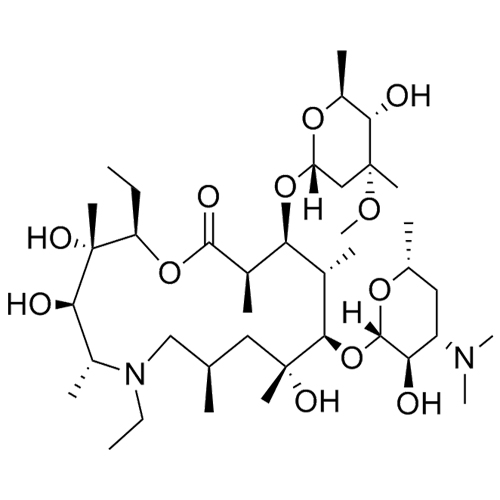
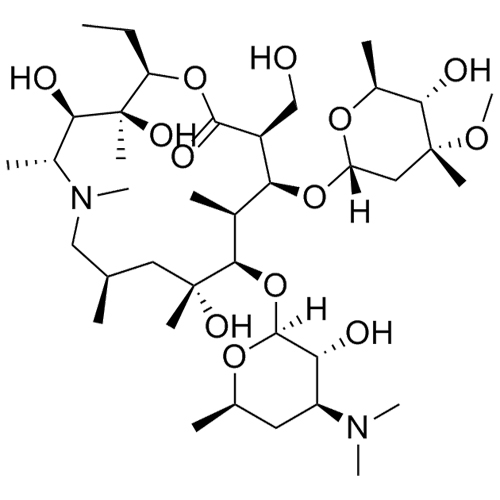
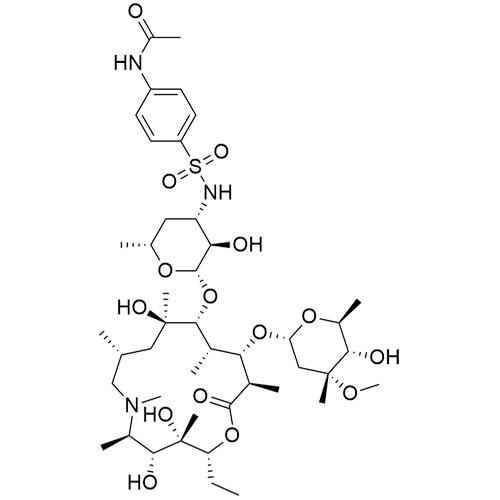
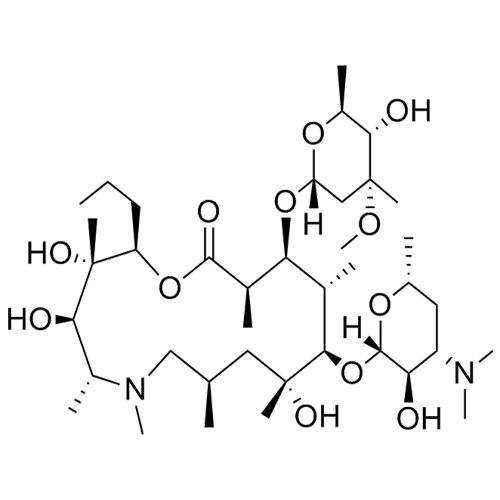
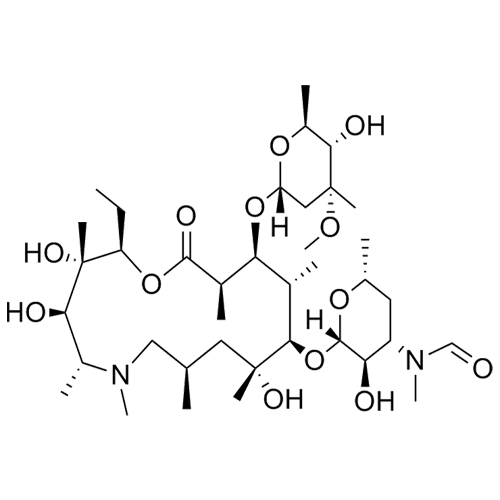
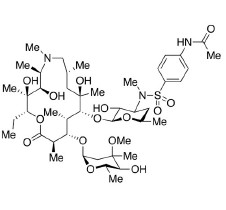
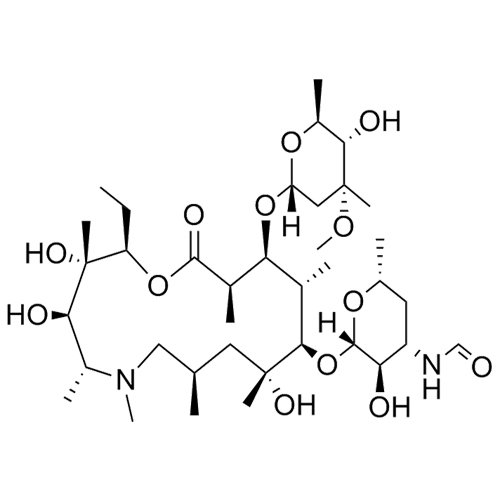
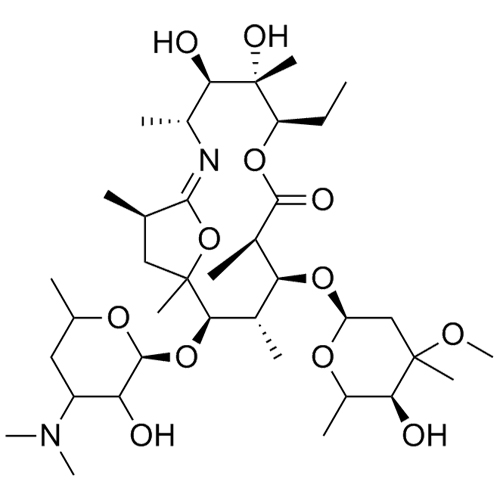
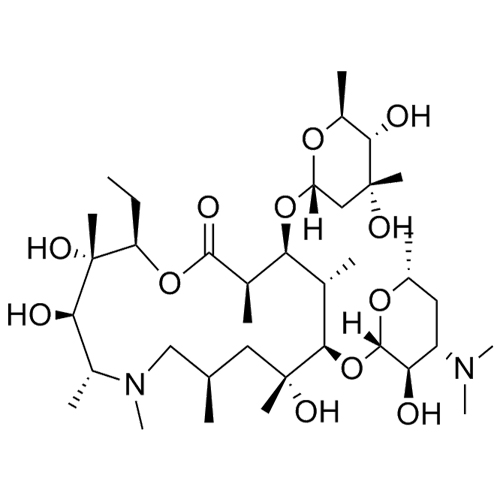
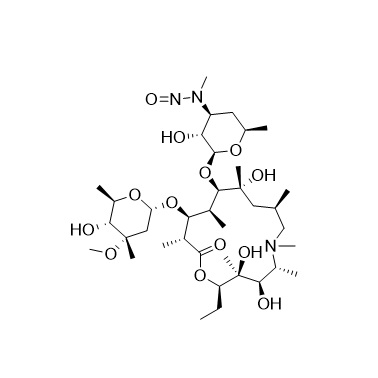
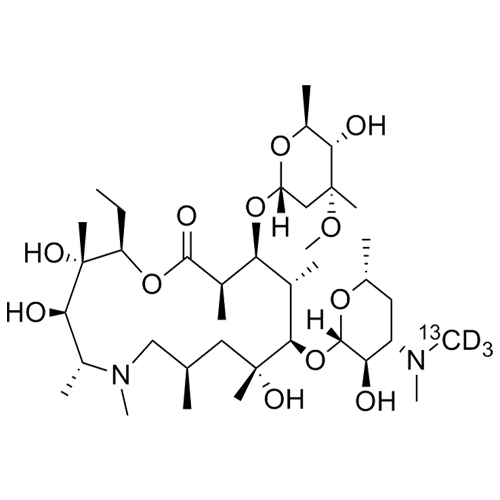
- SynonymsErythromycin A 6,9-Imino Ether; [3R-(3R*,4R*,5S*,6R*,9R*,10S*,11S*,12R*,13R*,15R*)]-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-?-L-ribo-hexopyranosyl)oxy]-6-ethyl-4,5-dihydroxy-3,5,9,11,13,15-hexamethyl-12-[[3,4,6-trideoxy-3-(dimethylamino)-?-D-xylo-hexopyranosyl]oxy]-16-Dioxa-2-azabicyclo[11.2.1]hex...
- Description
Erythromycin A 6,9-Imino Ether; [3R-(3R*,4R*,5S*,6R*,9R*,10S*,11S*,12R*,13R*,15R*)]-10-[(2,6-dideoxy-3-C-methyl-3-O-methyl-?-L-ribo-hexopyranosyl)oxy]-6-ethyl-4,5-dihydroxy-3,5,9,11,13,15-hexamethyl-12-[[3,4,6-trideoxy-3-(dimethylamino)-?-D-xylo-hexopyranosyl]oxy]-16-Dioxa-2-azabicyclo[11.2.1]hexadec-1-en-8-one
Erythromycin A (6,9-Imino Ether) is a fully characterized chemical compound used as a reference standard of API Azithromycin. The standard offered is compliant with regulatory guidelines. Erythromycin A (6,9-Imino Ether) is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 99290-97-8
Related products
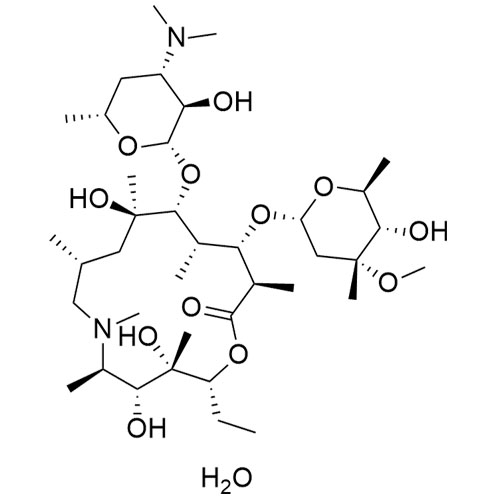
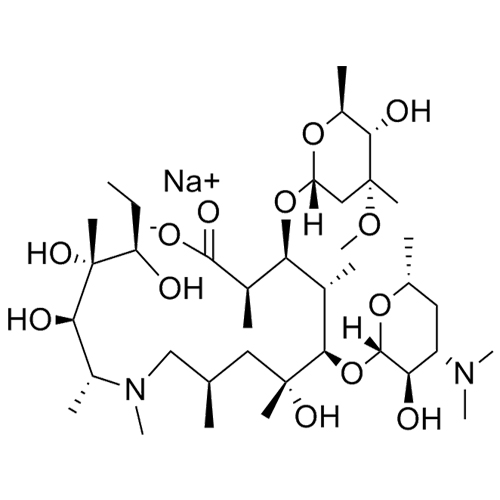
3-Hydroxy Azithromycinoic Acid Sodium Salt
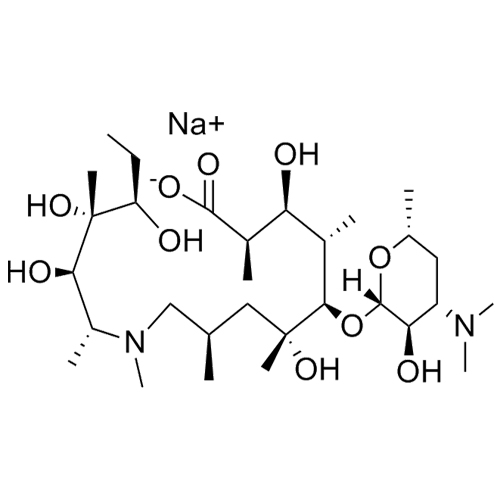
M.F. C₃₀H₅₉N₂O₁₀. Na
M.W. 607.81 22.99
CAT# AR-A03203
CAS# NA
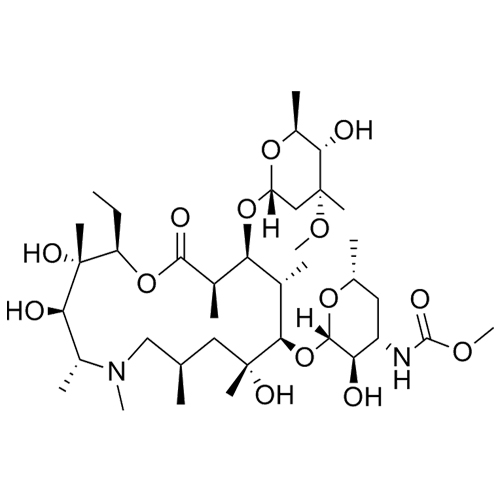
3’-N-Demethyl-3’-N- (phenylsulfonyl) azithromycin
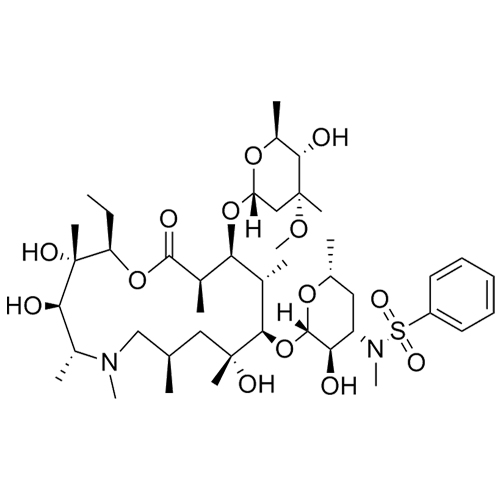
M.F. C₄₃H₇₄N₂O₁₄S
M.W. 875.13
CAT# AR-A03208
CAS# NA
Azithromycin Impurity 6 (Azithromycin 11,12-hydrogenborate)
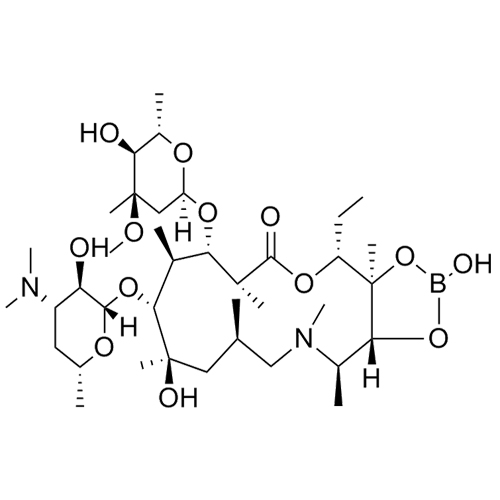
M.F. C₃₈H₇₁BN₂O₁₃
M.W. 774.8
CAT# AR-A03211
CAS# NA
Azaerythromycin A 11,12-hydrogen borate
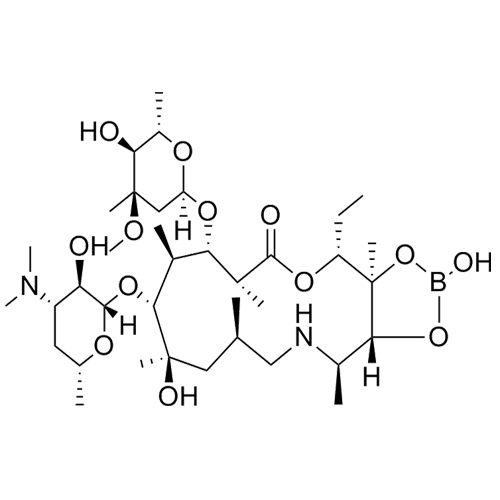
M.F. C₃₇H₆₉BN₂O₁₃
M.W. 760.77
CAT# AR-A03207
CAS# 194809-66-0
Azithromycin EP Impurity L (Azithromycin N-Oxide)
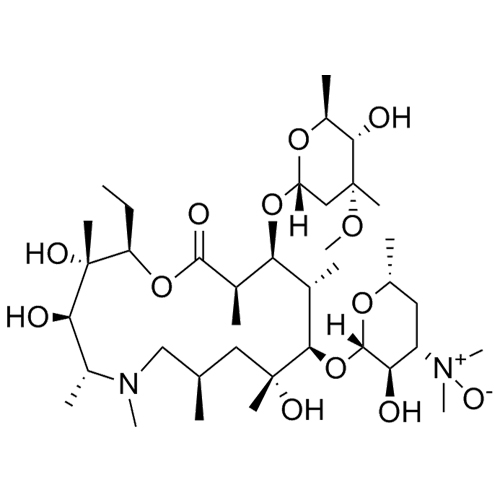
M.F. C₃₈H₇₂N₂O₁₃
M.W. 765
CAT# AR-A03188
CAS# 90503-06-3
Azithromycin EP Impurity I (N-Desmethyl Azithromycin)

M.F. C₃₇H₇₀N₂O₁₂
M.W. 734.97
CAT# AR-A03185
CAS# 172617-84-4