- Synonyms(2RS)-2-[4-[2-(diethylamino)ethoxy]phenyl]-1,2-diphenylethan-1-one Hydrochloride (as per EP) ; 2-{4-[2-(Diethylamino)ethoxy]phenyl}-1,2-diphenylethan-1-one (as per USP)
- Description
(2RS)-2-[4-[2-(diethylamino)ethoxy]phenyl]-1,2-diphenylethan-1-one Hydrochloride (as per EP) ; 2-{4-[2-(Diethylamino)ethoxy]phenyl}-1,2-diphenylethan-1-one (as per USP)
Clomiphene EP Impurity C HCl is a fully characterized chemical compound used as a reference standard of API Clomiphene. The standard offered is compliant with regulatory guidelines. Clomiphene EP Impurity C HCl is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 5635-70-1
Related products
N-Desethyl Clomiphene HCl (Mixture of Z and E Isomers)
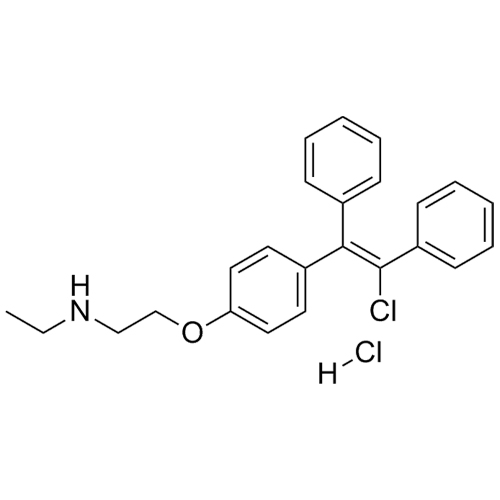
M.F.
M.W. 377.92 36.46
CAT# AR-C03199
CAS# 1310815-19-0
1-(4-(2-(diethylamino)ethoxy)phenyl)-1,2-diphenylethanol

M.F.
M.W. 389.54
CAT# AR-C03200
CAS# 73404-00-9
Clomiphene EP Impurity G, H Citrate (Mixture of Z and E Isomers)
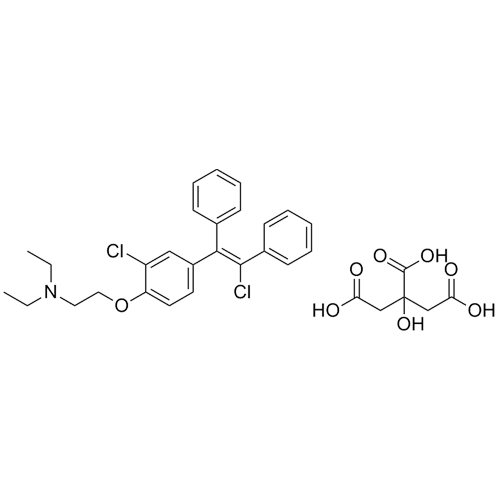
M.F.
M.W. 440.42 192.13
CAT# AR-C03197
CAS# 1795130-18-5
Clomiphene Impurity 8 HCl (Mixture of Z and E Isomers)

M.F.
M.W. 496.09 36.46
CAT# AR-C08067
CAS# NA
Clomiphene Impurity 8 Citrate (Mixture of Z and E Isomers)

M.F.
M.W. 496.09 192.12
CAT# AR-C08066
CAS# NA
trans-Clomiphene-d5 HCl (Enclomiphene-d5 HCl)

M.F.
M.W. 411.00 36.46
CAT# AR-C03193
CAS# 14158-65-7 (non-labelled)
Clomiphene-d5 HCl (Mixture of Z and E Isomers)
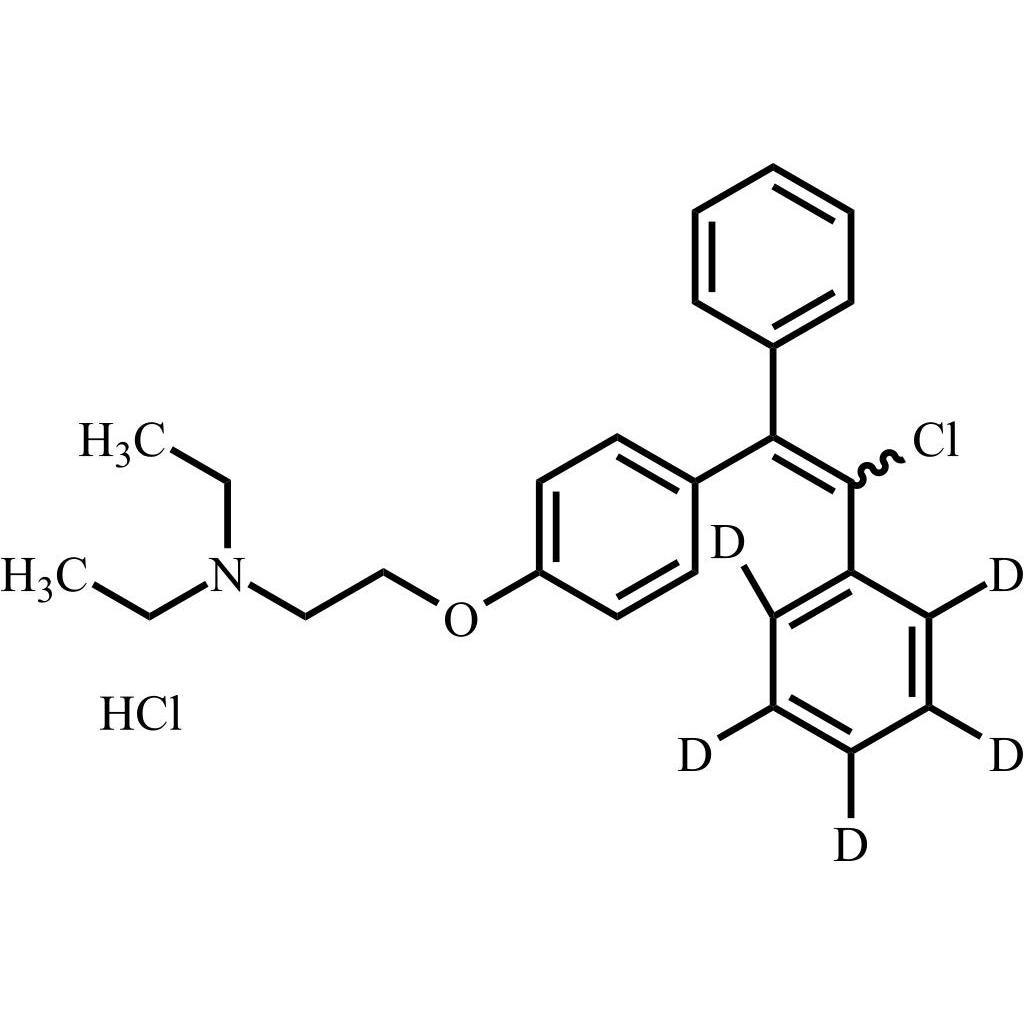
M.F.
M.W. 411.00 36.46
CAT# AR-C08071
CAS# 1346606-66-3