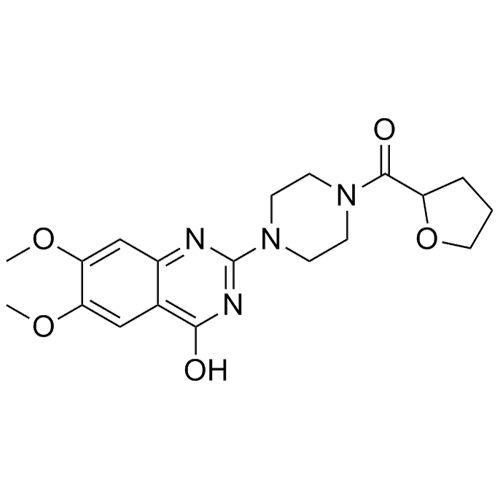
- Synonyms1-(4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl)-5-hydroxypentan-1-one; 1-[4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl]-5-hydroxy-1-pentanone
- Description
1-(4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl)-5-hydroxypentan-1-one; 1-[4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl]-5-hydroxy-1-pentanone
Terazosin EP Impurity F is a fully characterized chemical compound used as a reference standard of API Terazosin. The standard offered is compliant with regulatory guidelines. Terazosin EP Impurity F is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 109678-71-9
Related products
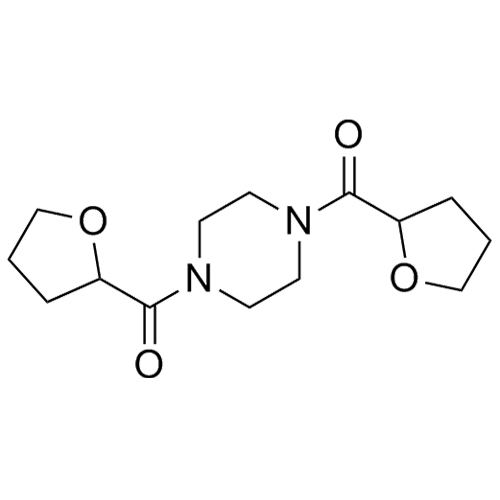
Terazosin EP Impurity L HCl (Prazosin EP Impurity D HCl)
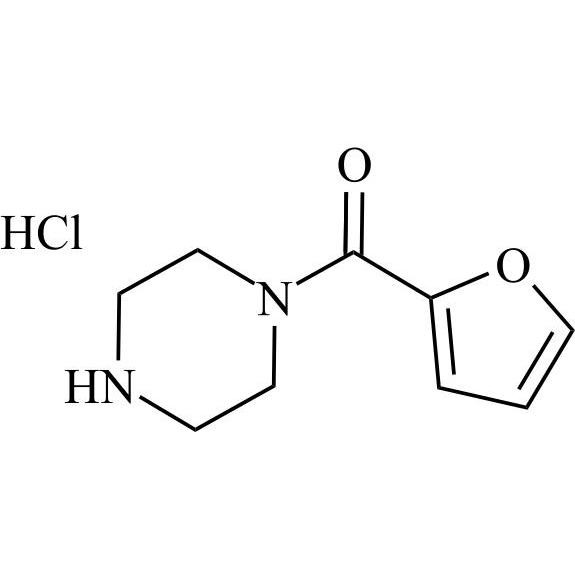
M.F.
M.W. 180.21 36.46
CAT# AR-T04407
CAS# 60548-09-6
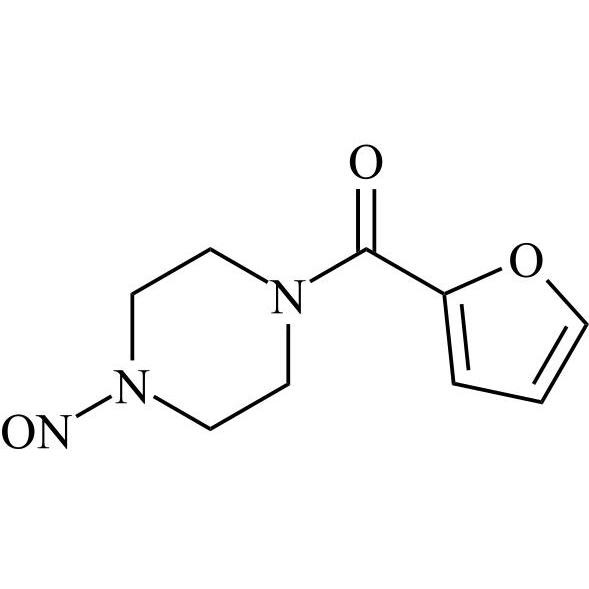
N-Nitroso Terazosin EP Impurity C (N-Nitroso Terazosin USP Related Compound A (Free Form), N-Nitroso Doxazosin EP Impurity G, N-Nitroso Prazosin EP Impurity C)
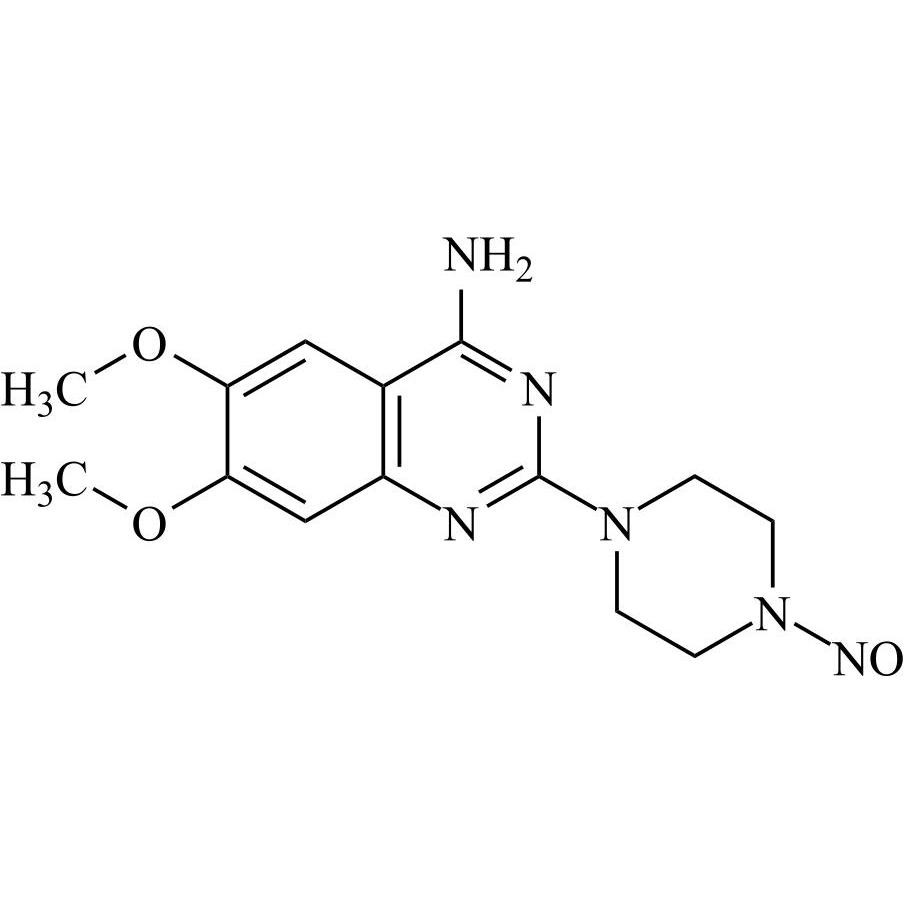
M.F.
M.W. 318.34
CAT# AR-T04406
CAS# NA