- Synonyms1-(4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl)-2-hydroxypentan-1-one; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-hydroxy-1-oxopentyl)piperazine; A 65297; (±)-1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-hydroxy-1-oxopentyl)piperazine
- Description
1-(4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl)-2-hydroxypentan-1-one; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-hydroxy-1-oxopentyl)piperazine; A 65297; (±)-1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-hydroxy-1-oxopentyl)piperazine
Terazosin EP Impurity J is a fully characterized chemical compound used as a reference standard of API Terazosin. The standard offered is compliant with regulatory guidelines. Terazosin EP Impurity J is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 152551-75-2
Related products
Terazosin EP Impurity L HCl (Prazosin EP Impurity D HCl)
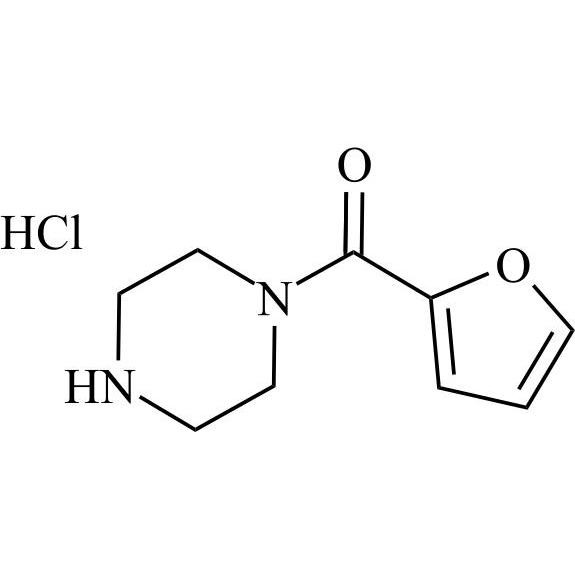
M.F.
M.W. 180.21 36.46
CAT# AR-T04407
CAS# 60548-09-6
N-Nitroso Terazosin EP Impurity C (N-Nitroso Terazosin USP Related Compound A (Free Form), N-Nitroso Doxazosin EP Impurity G, N-Nitroso Prazosin EP Impurity C)
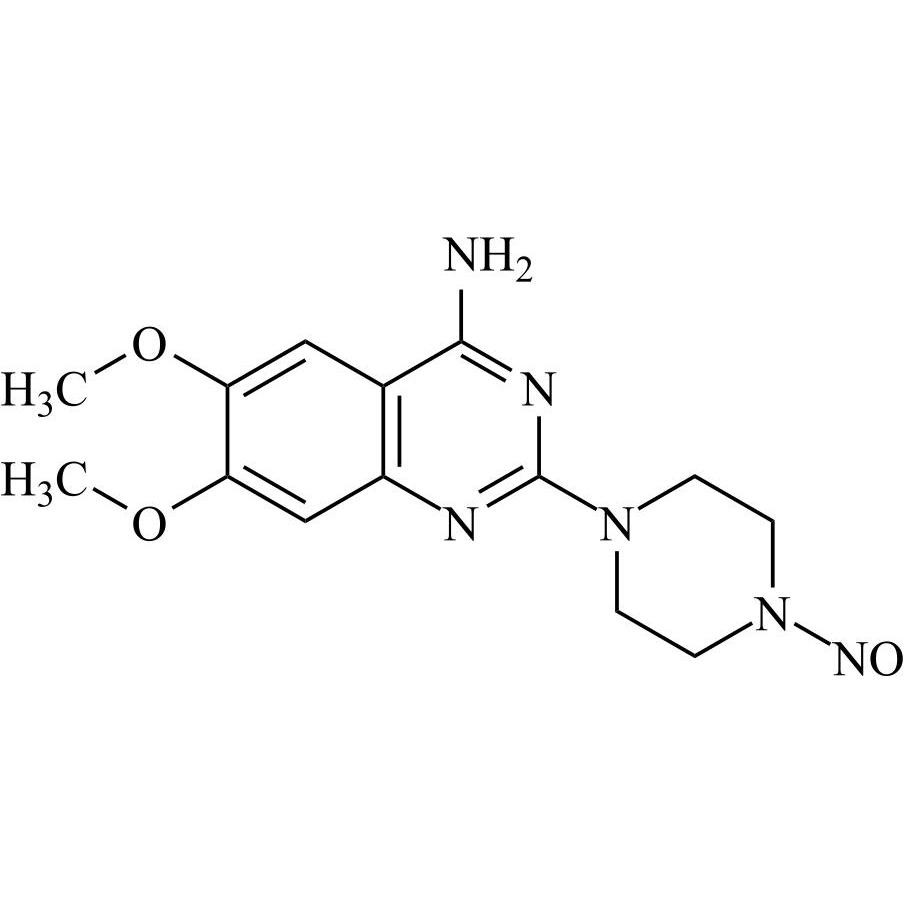
M.F.
M.W. 318.34
CAT# AR-T04406
CAS# NA