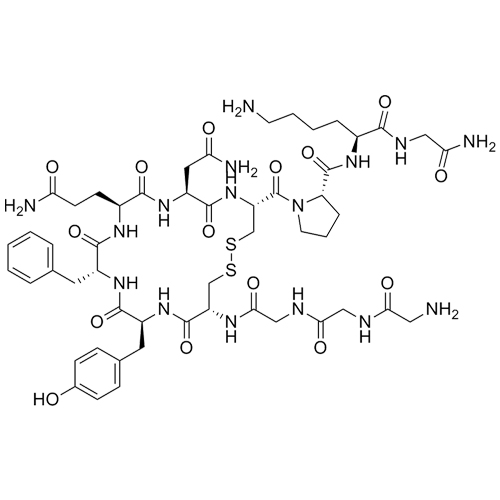
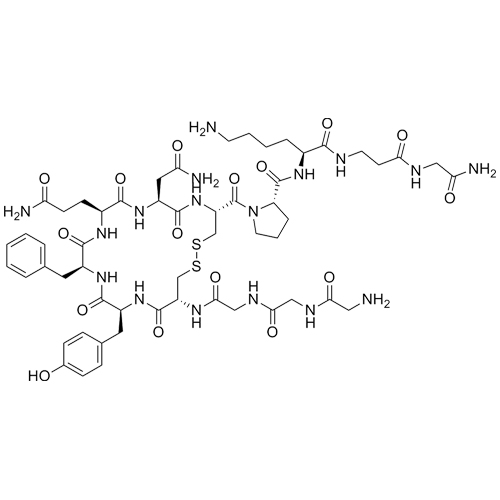
(S)-N-((S)-6-amino-1-((2-amino-2-oxoethyl)amino)-1-oxohexan-2-yl)-1-((4R,7S,10S,13S,16S,19R)-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-19-(2-aminoacetamido)-13-benzyl-16-(4-hydroxybenzyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carbonyl)pyrrolidine-2-carboxamide; Des-1,2-Diglycine-Terlipressin; des(Gly(1-2))-Terlipressin; [des(Gly(1-2))-TLY], (2-7-cyclo)-H-Gly-Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Lys-Gly-NH2
Terlipressin EP Impurity B (TFA salt) is a fully characterized chemical compound used as a reference standard of API Terlipressin. The standard offered is compliant with regulatory guidelines. Terlipressin EP Impurity B (TFA salt) is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - NA