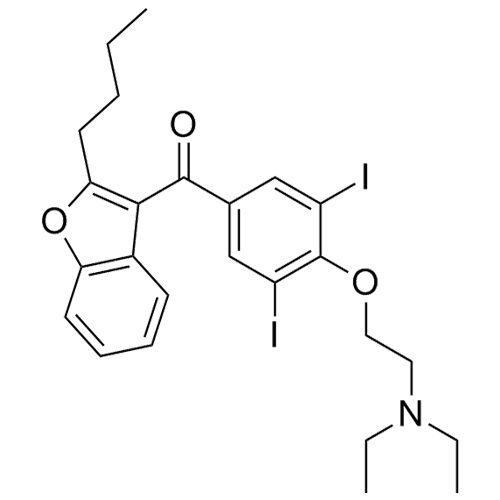
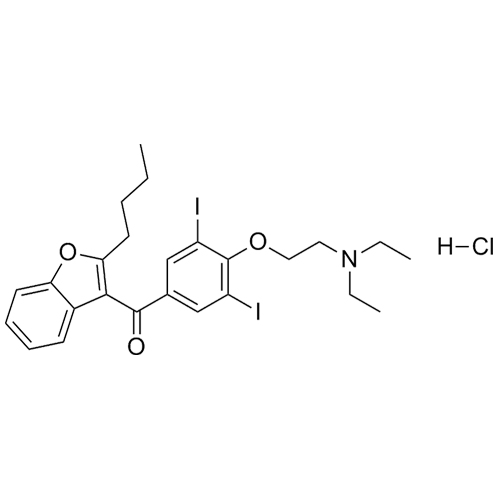
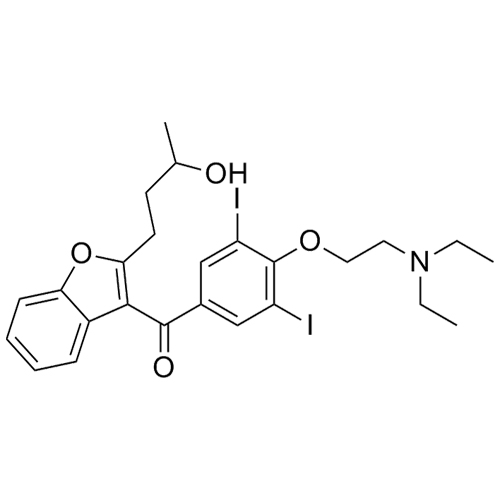
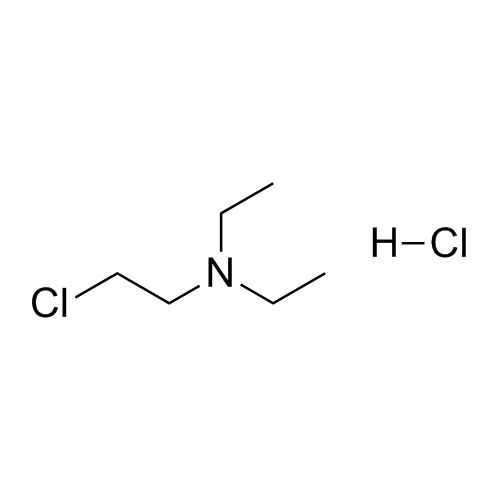
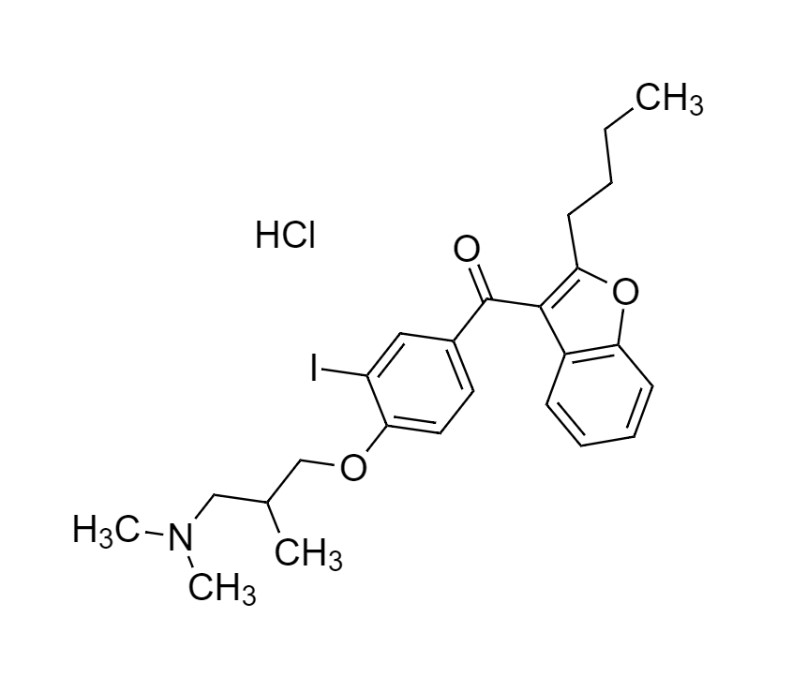
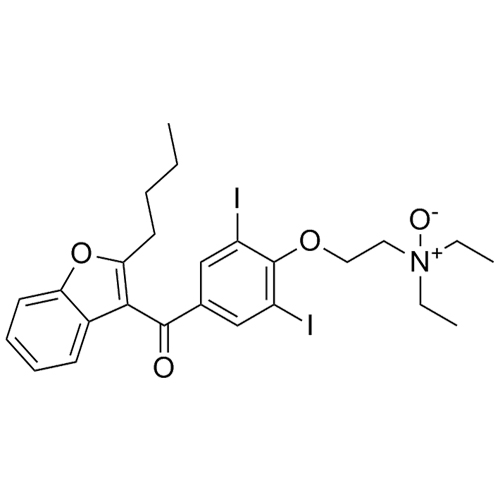
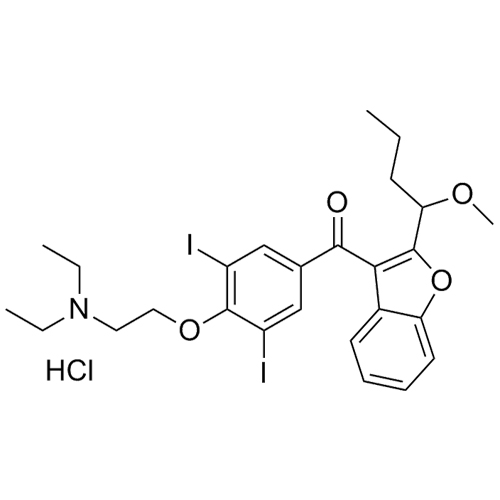
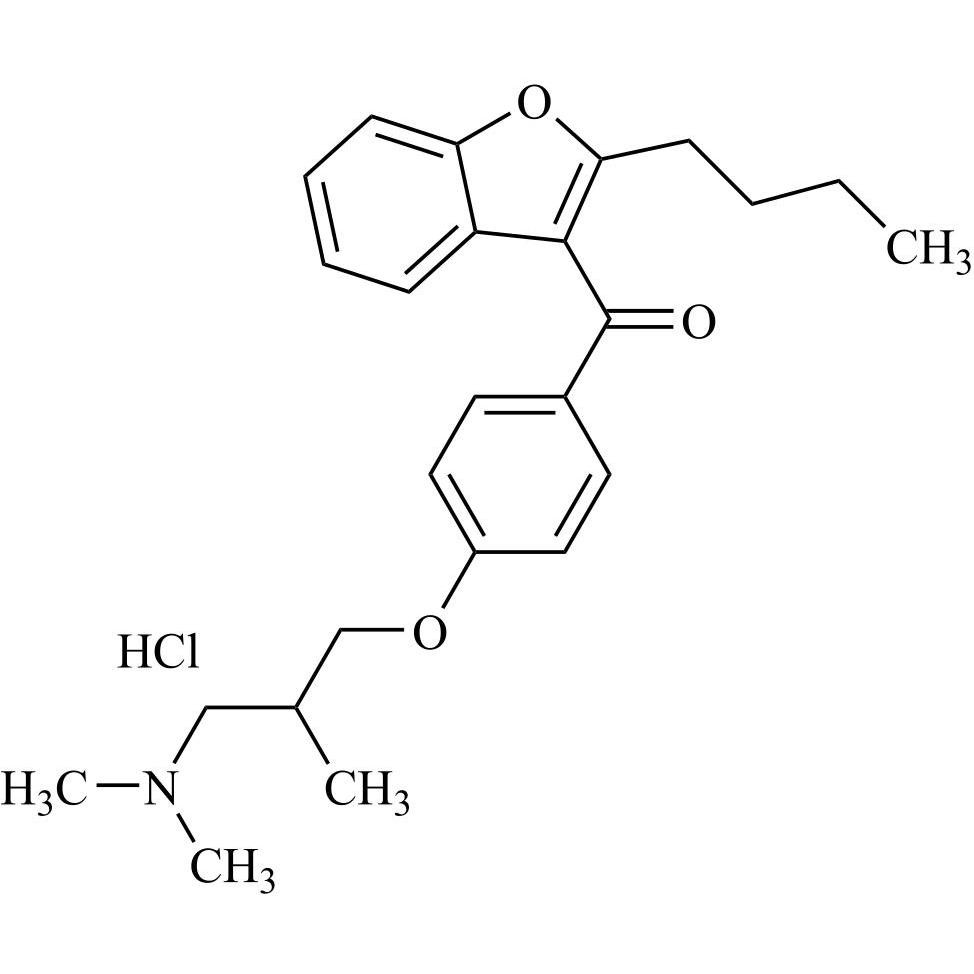
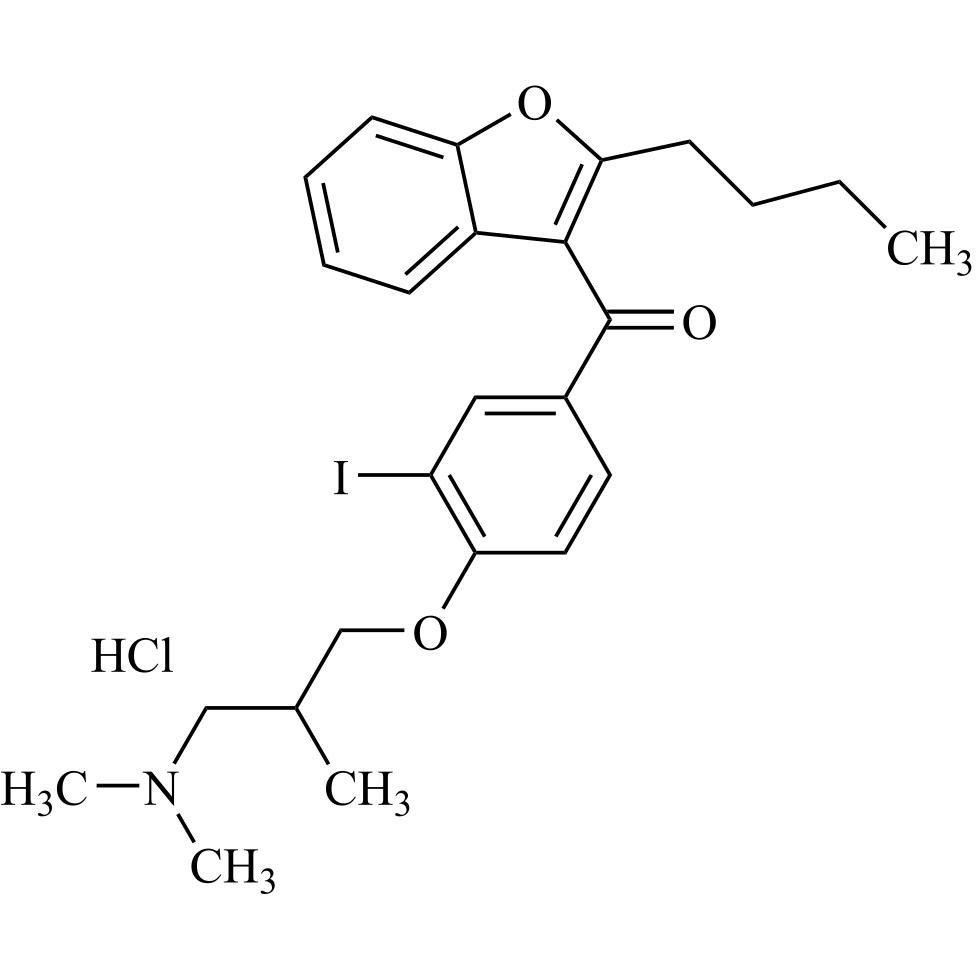
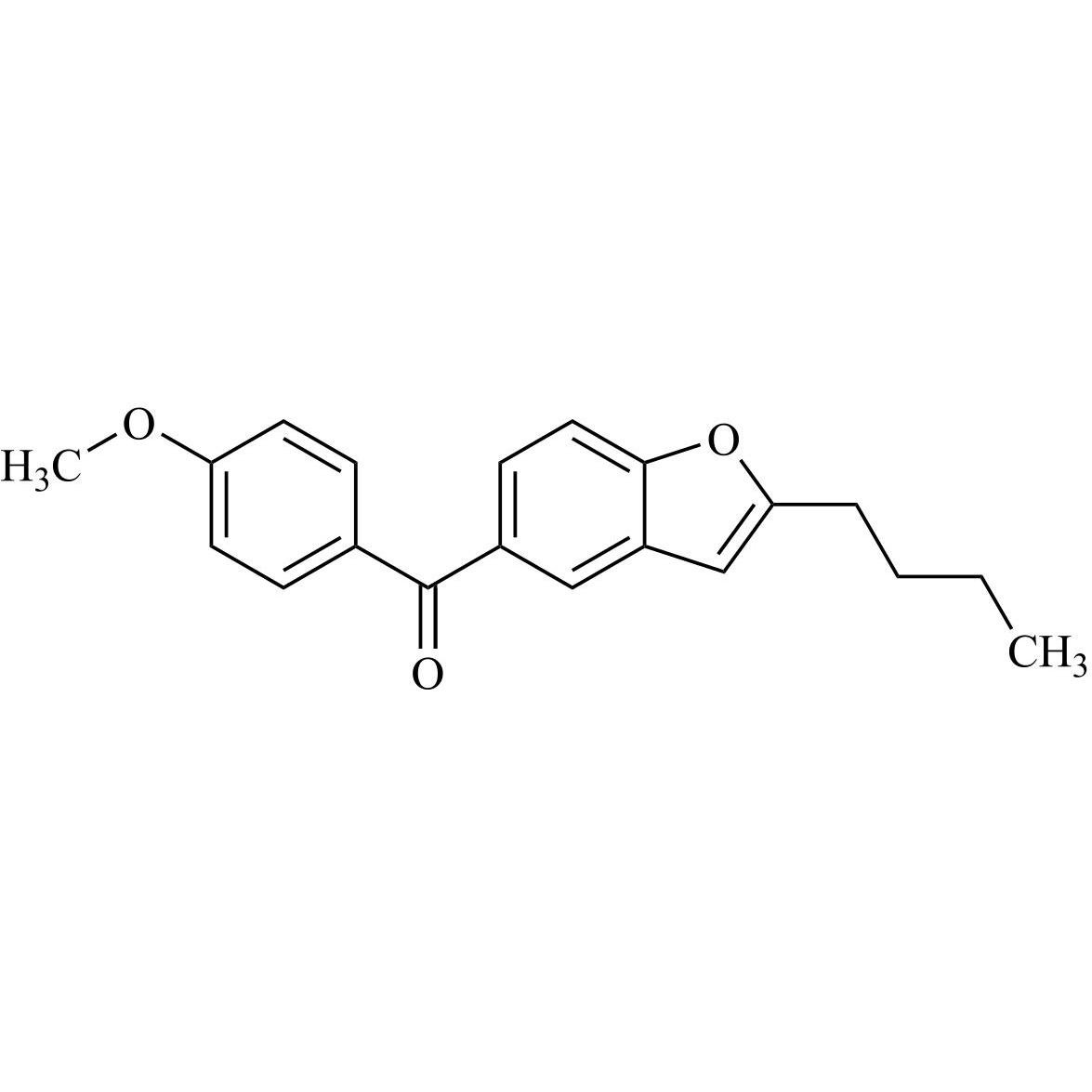
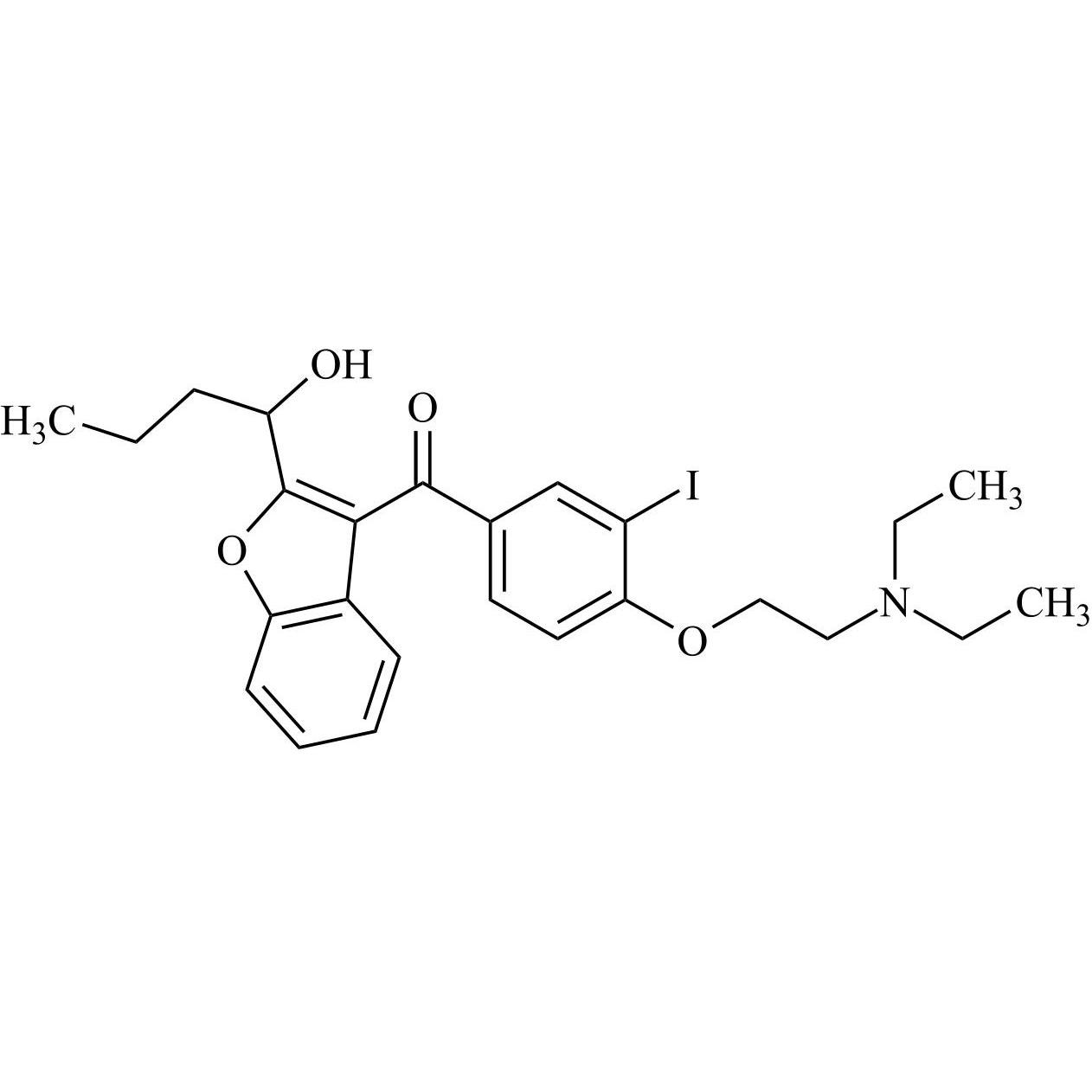
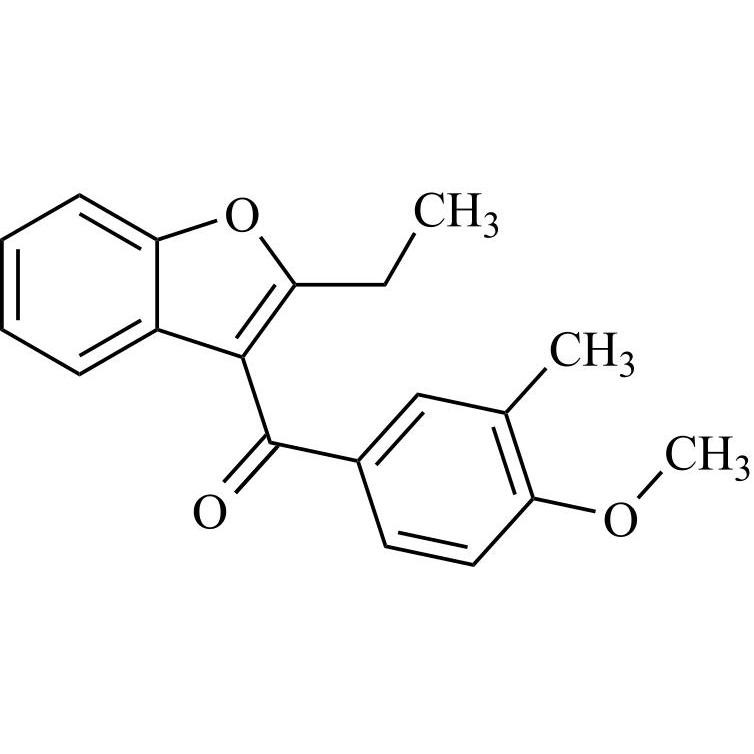
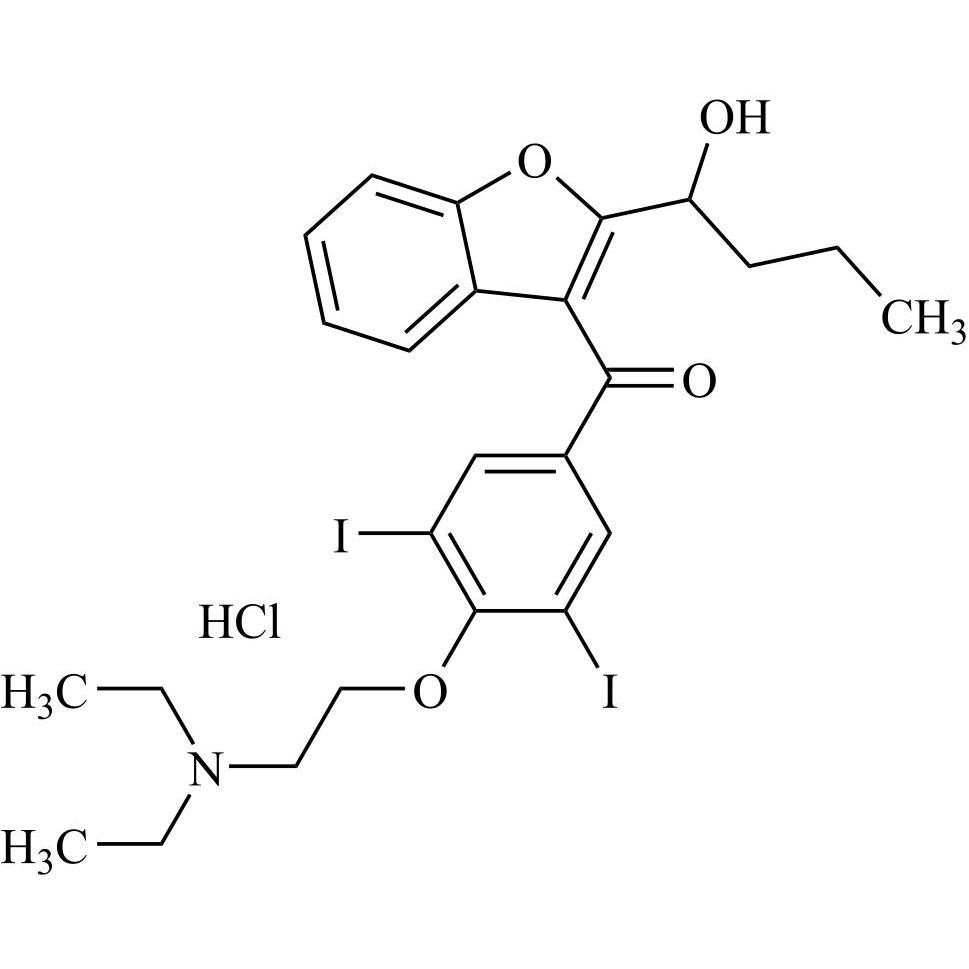
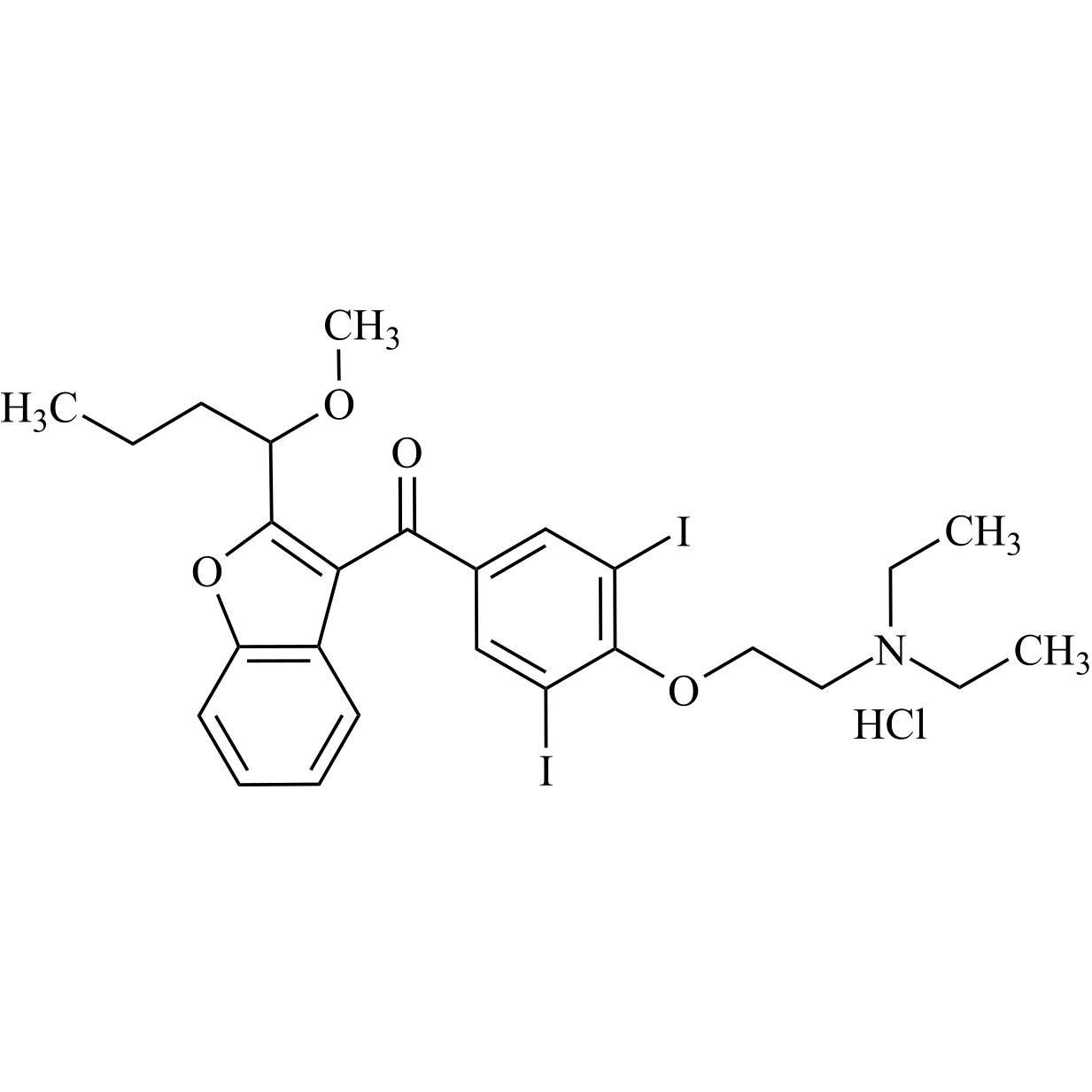
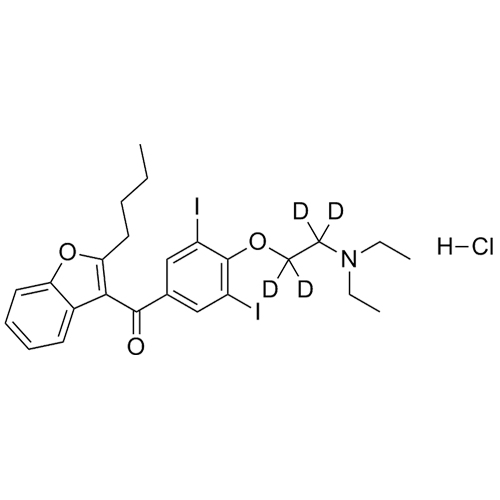
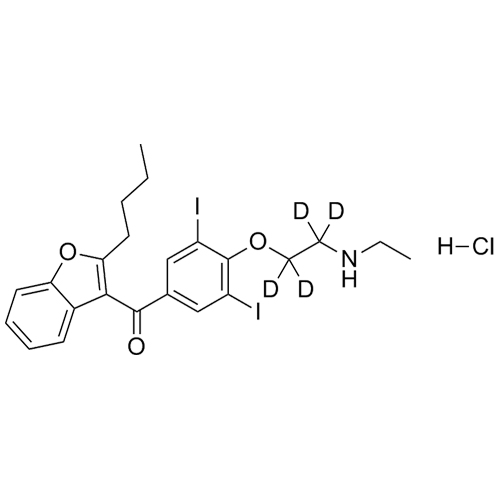
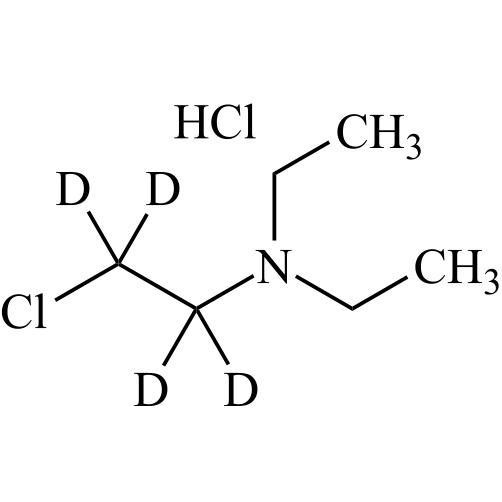
- Synonyms(2-butylbenzofuran-3-yl)(4-(2-(diethylamino)ethoxy)phenyl)methanone hydrochloride; (2-Butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]phenyl]methanone Hydrochloride; 2-Butyl-3-benzofuranyl p-[2-(diethylamino)ethoxy]phenylketone Hydrochloride; L 3937 Hydrochloride
- Description
(2-butylbenzofuran-3-yl)(4-(2-(diethylamino)ethoxy)phenyl)methanone hydrochloride; (2-Butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]phenyl]methanone Hydrochloride; 2-Butyl-3-benzofuranyl p-[2-(diethylamino)ethoxy]phenylketone Hydrochloride; L 3937 Hydrochloride
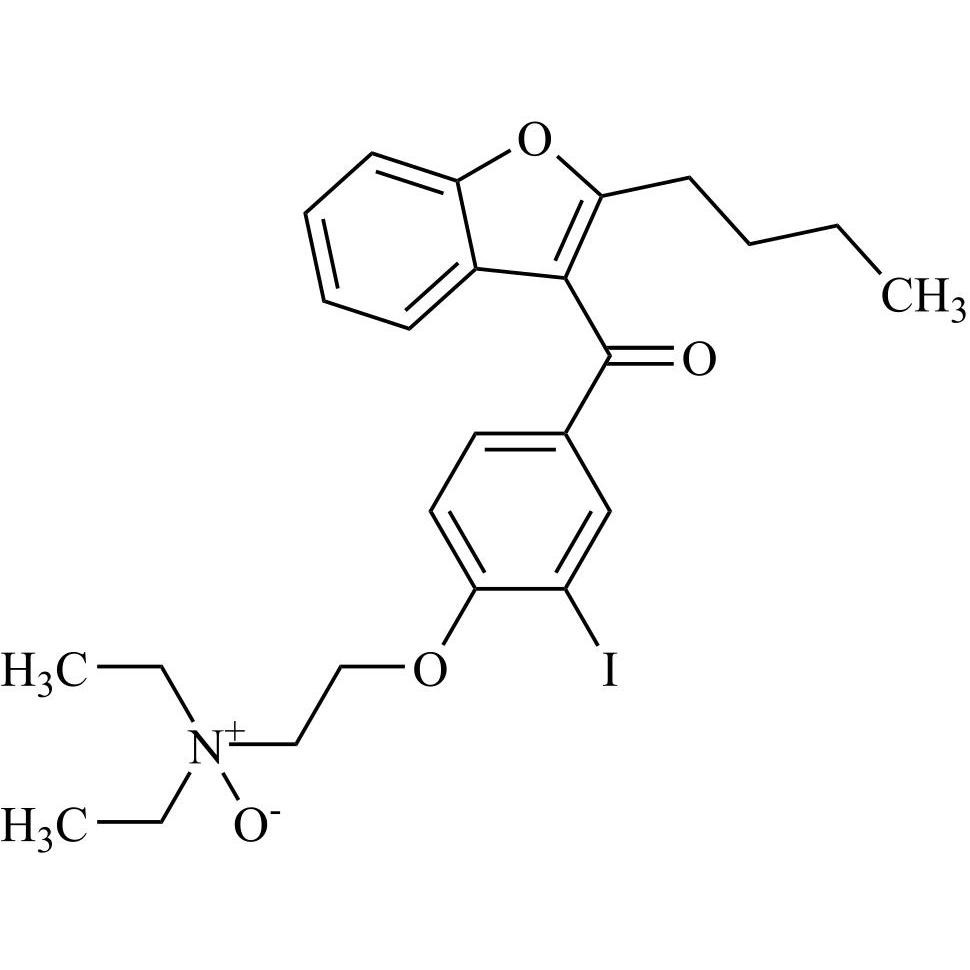
Amiodarone EP Impurity A HCl is a fully characterized chemical compound used as a reference standard of API Amiodarone. The standard offered is compliant with regulatory guidelines. Amiodarone EP Impurity A HCl is used for analytical method development, method validation (AMV), and Quality Controlled (QC) applications during synthesis and formulation stages of drug development and serves as a reference standard for traceability against pharmacopeial standards (USP or EP). Axios Research products are intended for analytical purposes only and are not for human use. CAS - 95820-13-6
Related products
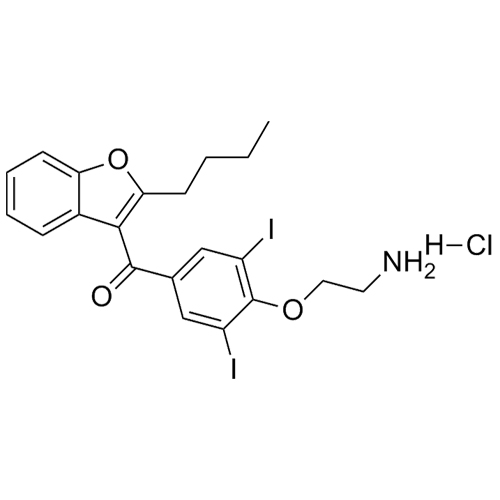
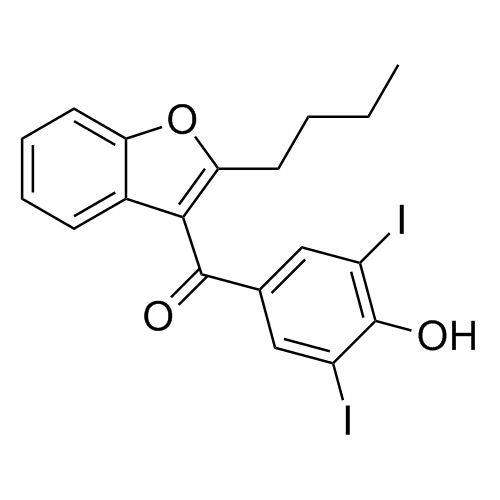
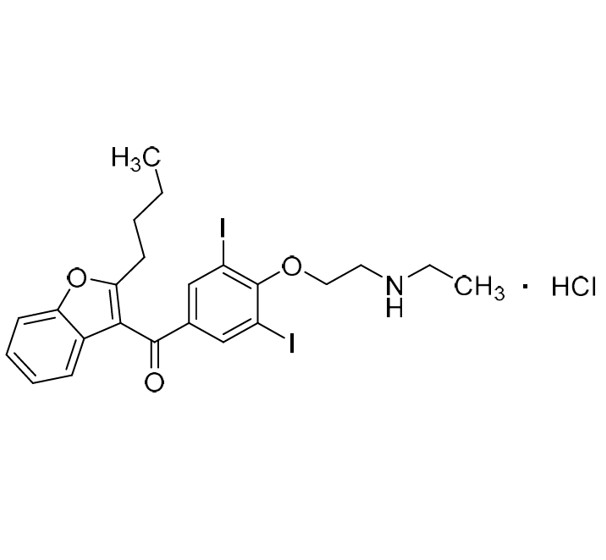
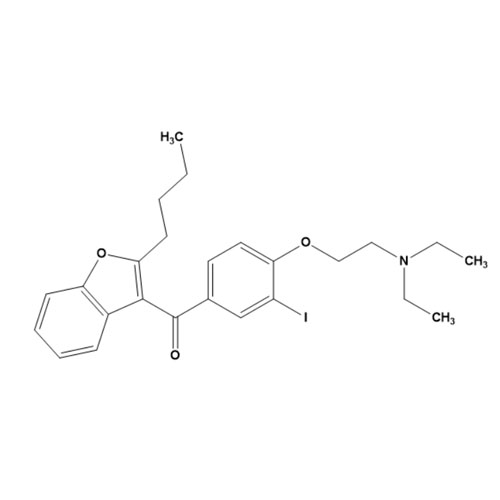
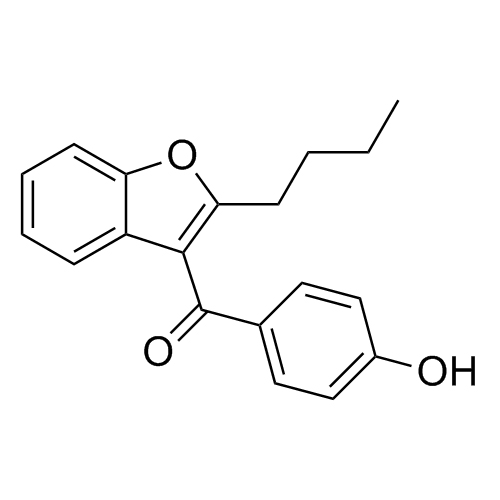
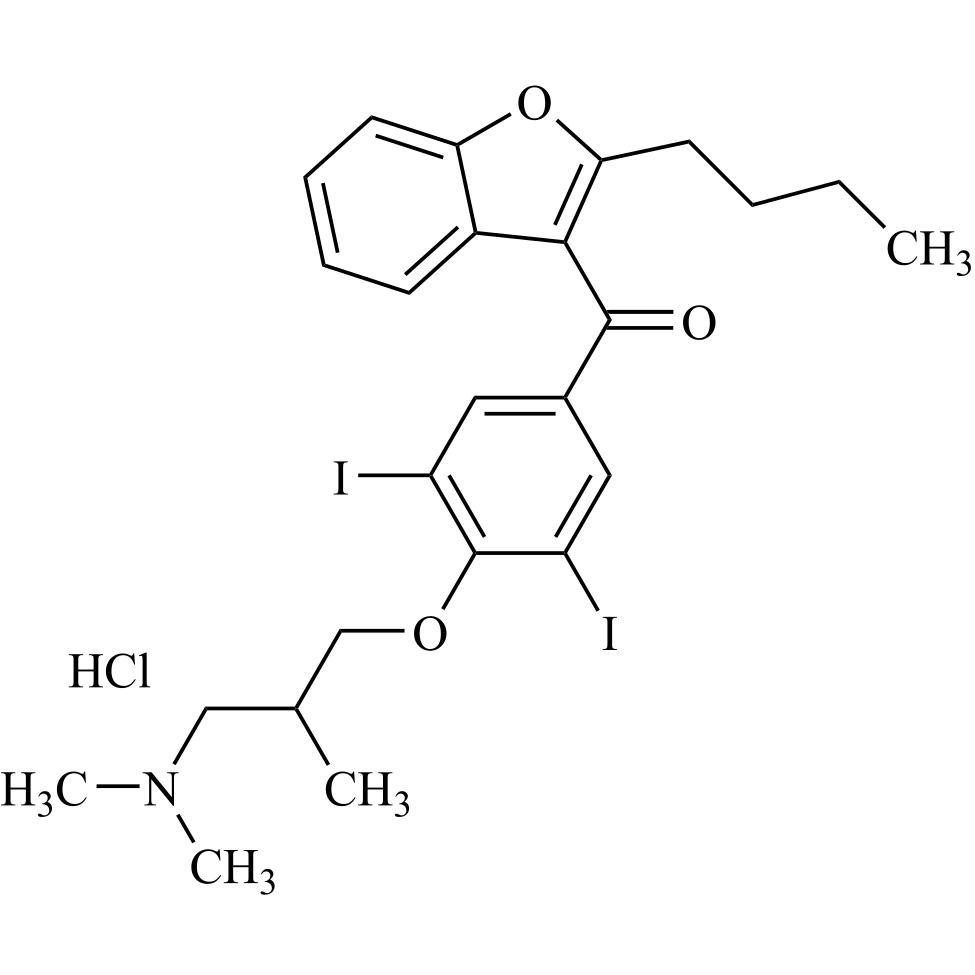
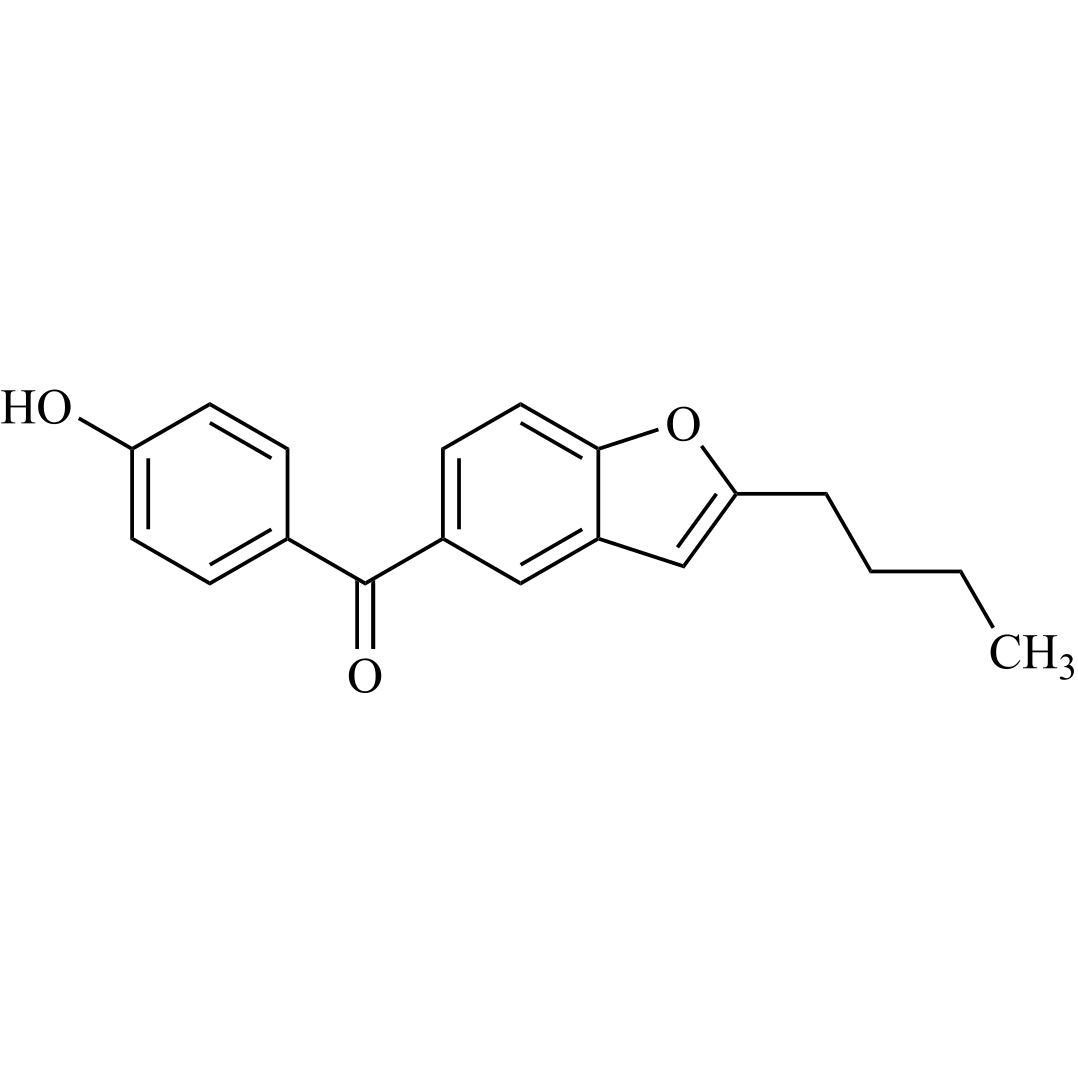
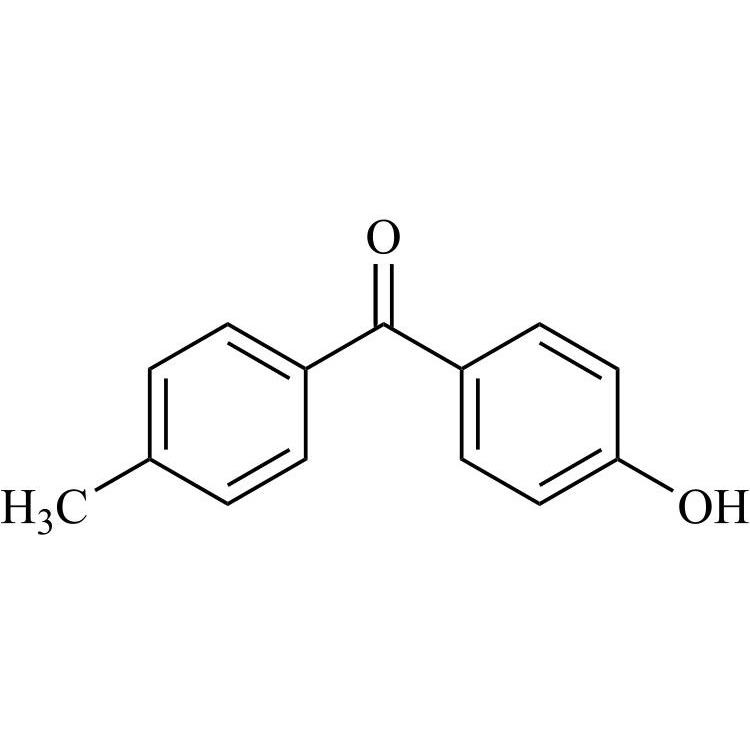
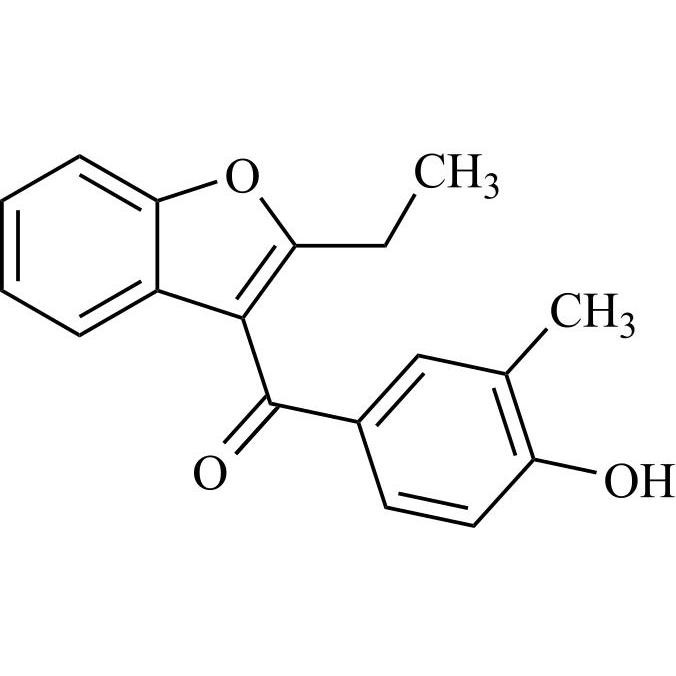
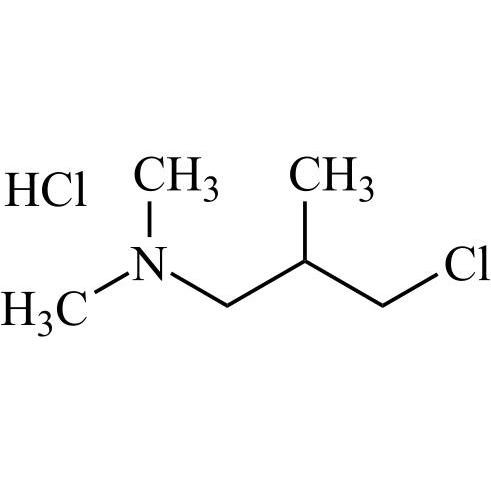
Amiodarone EP Impurity C HCl (Deiodo Impurity)

M.F.
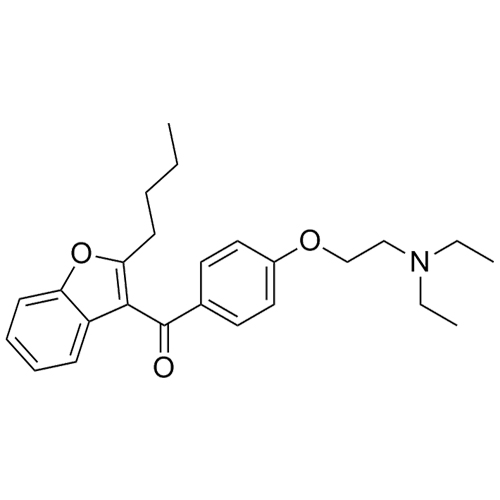
M.W. 519.42; 36.46
CAT# AR-A01940
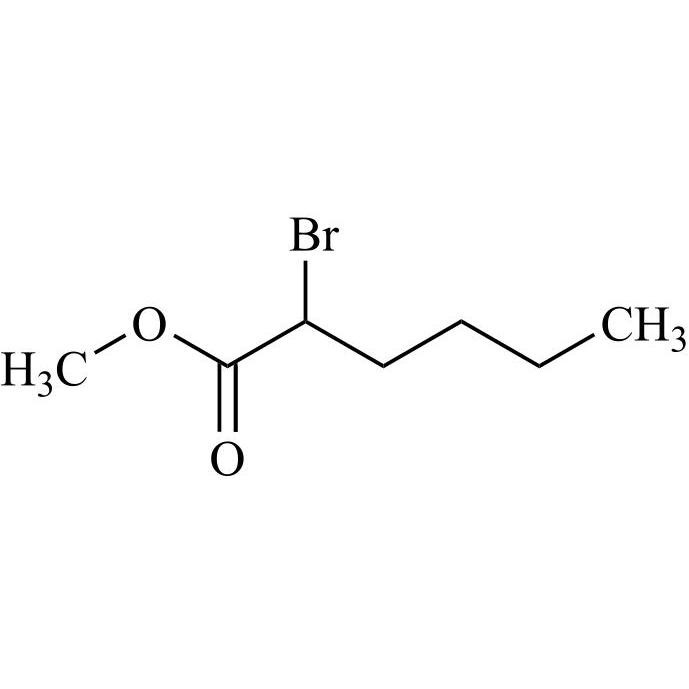
CAS# 1397201-93-2
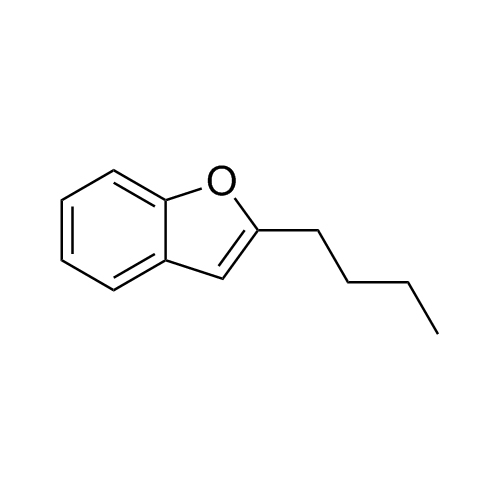
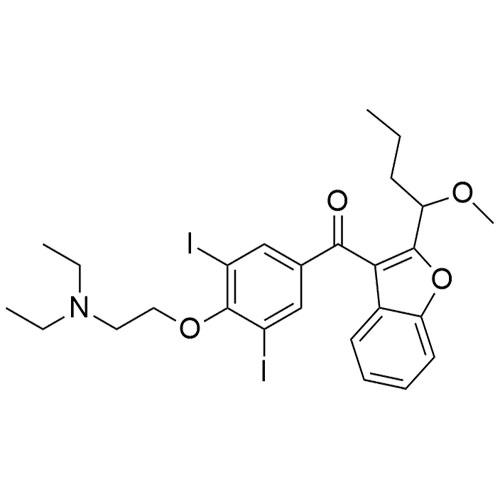
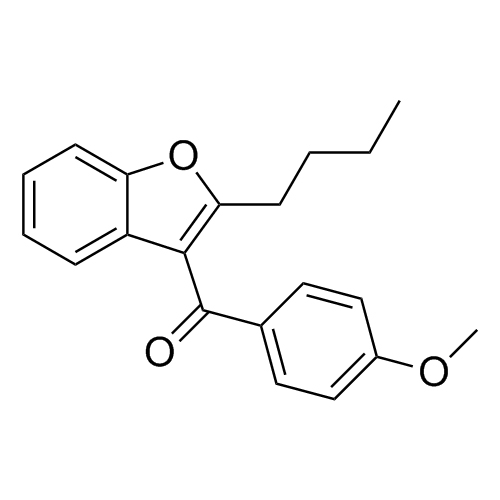
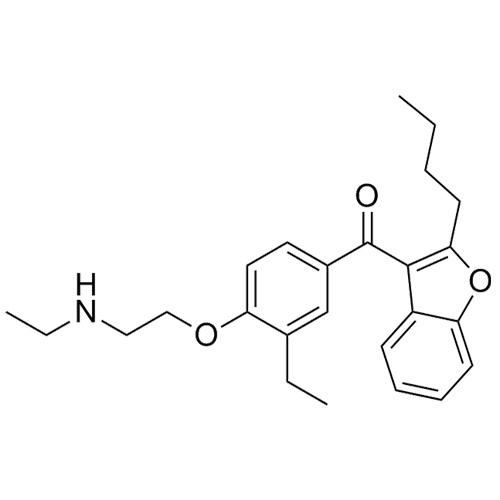
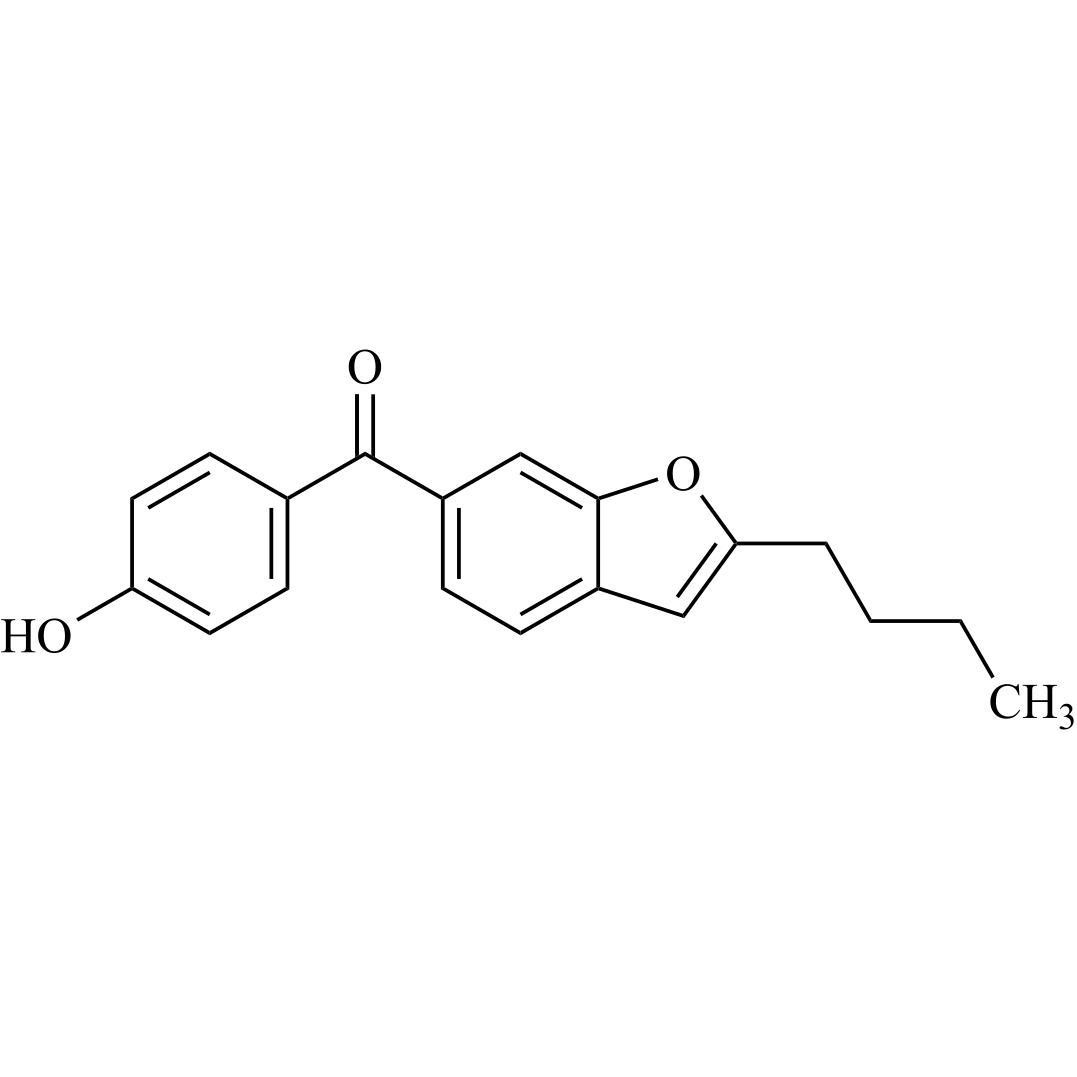
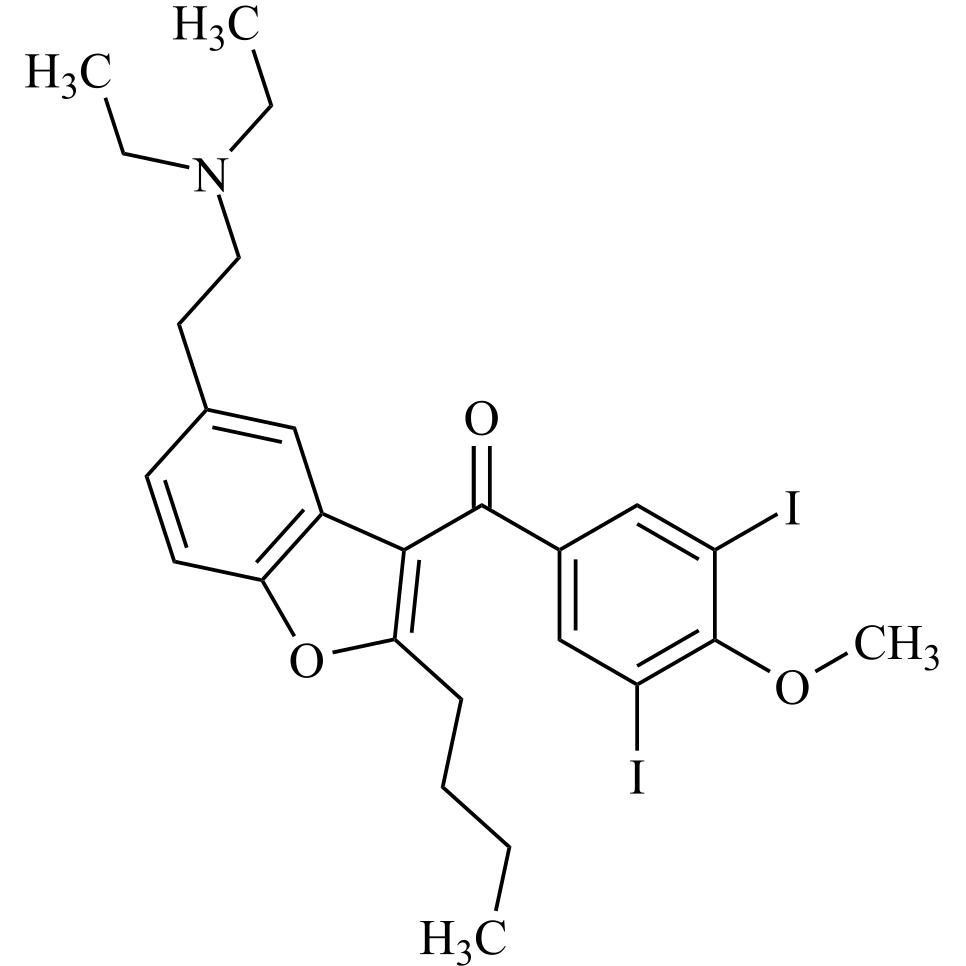
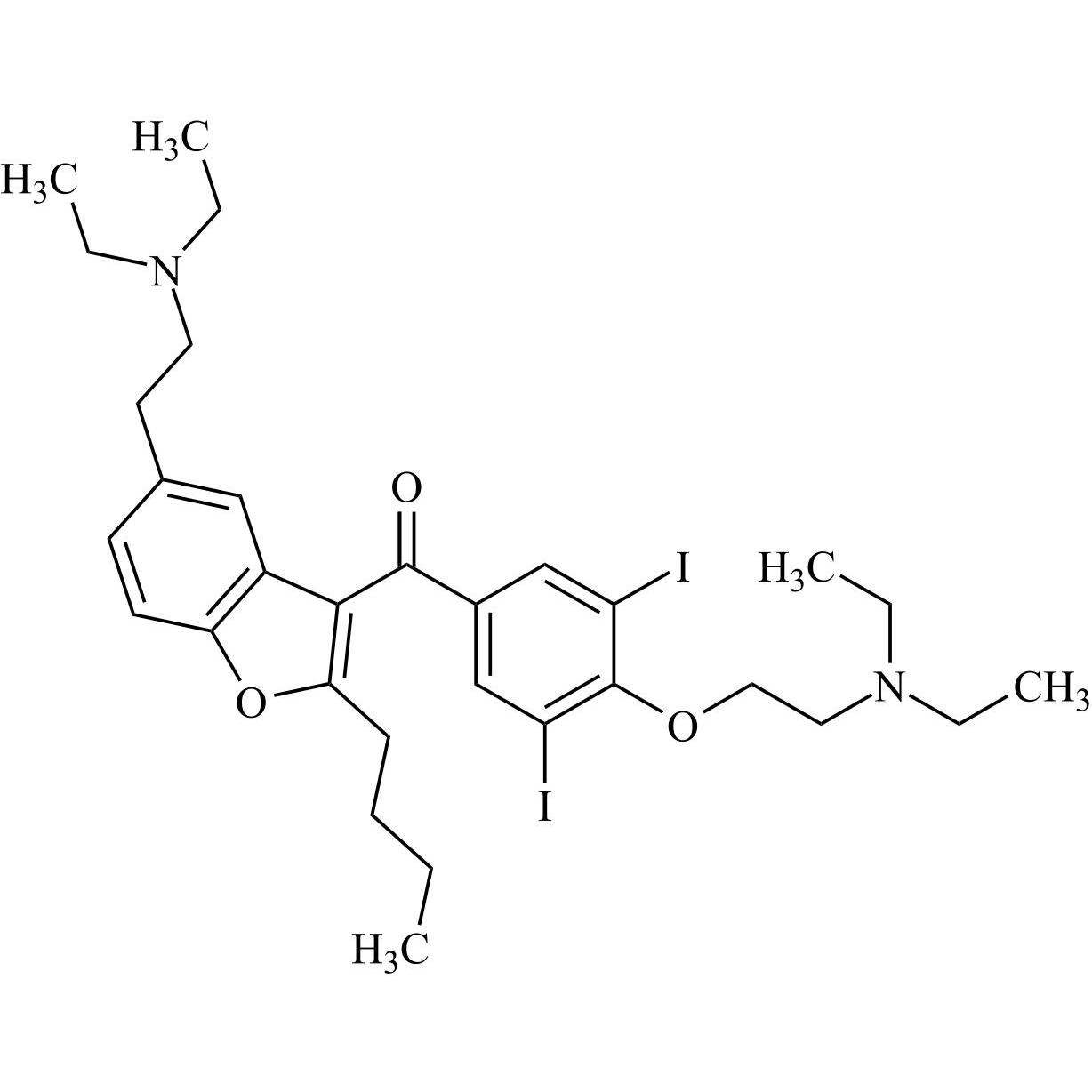
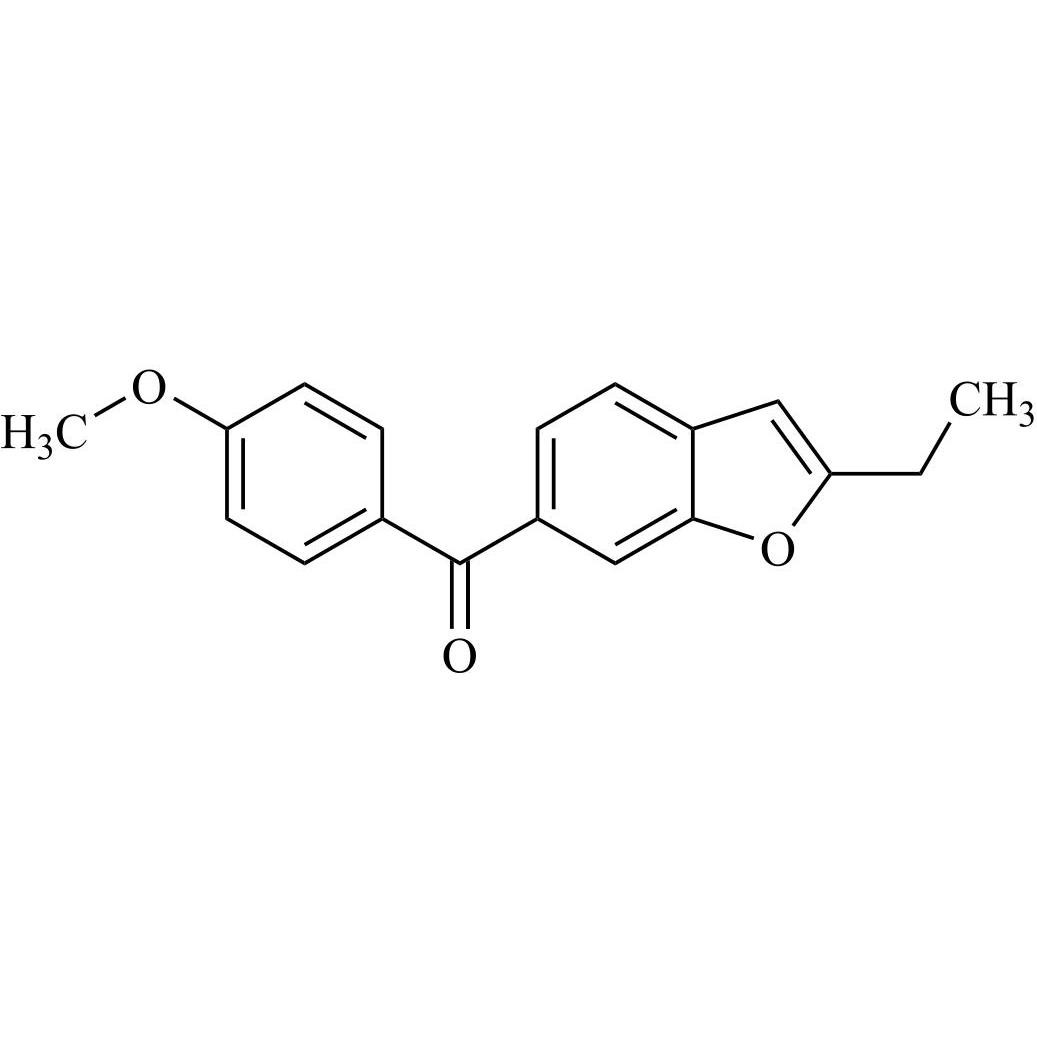
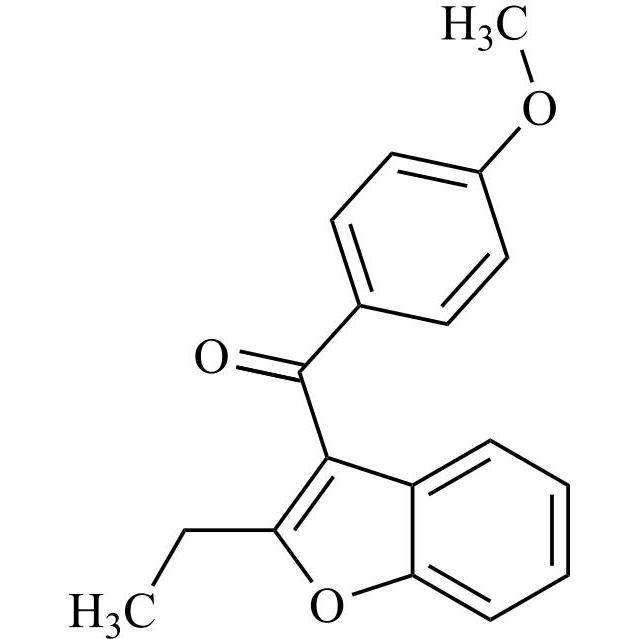
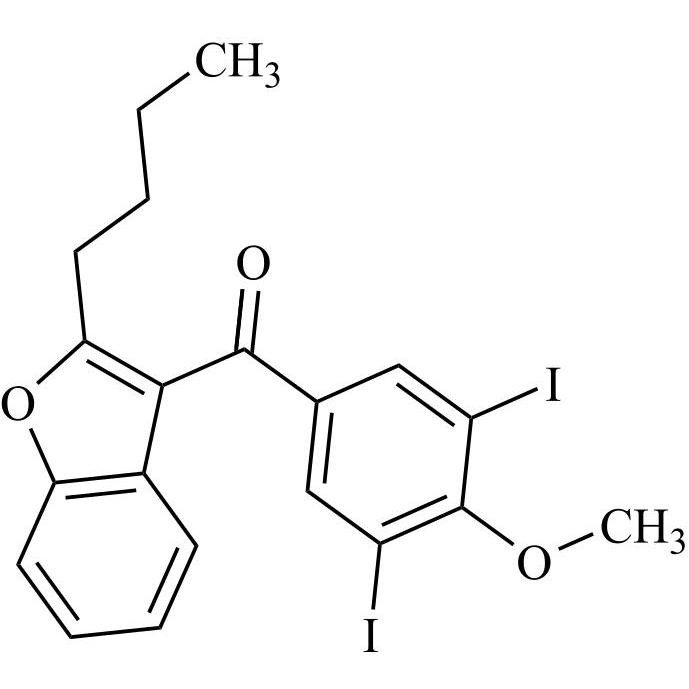
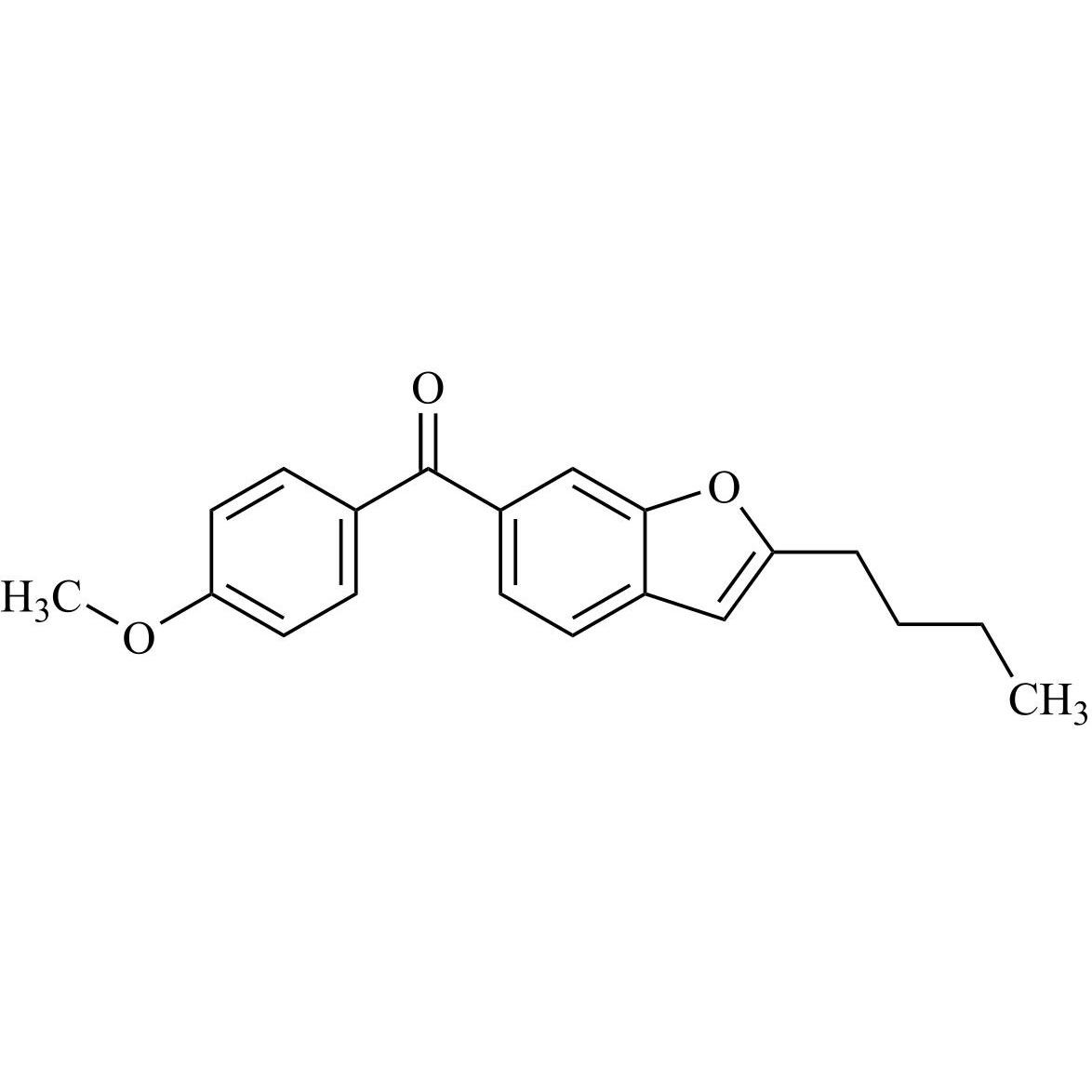
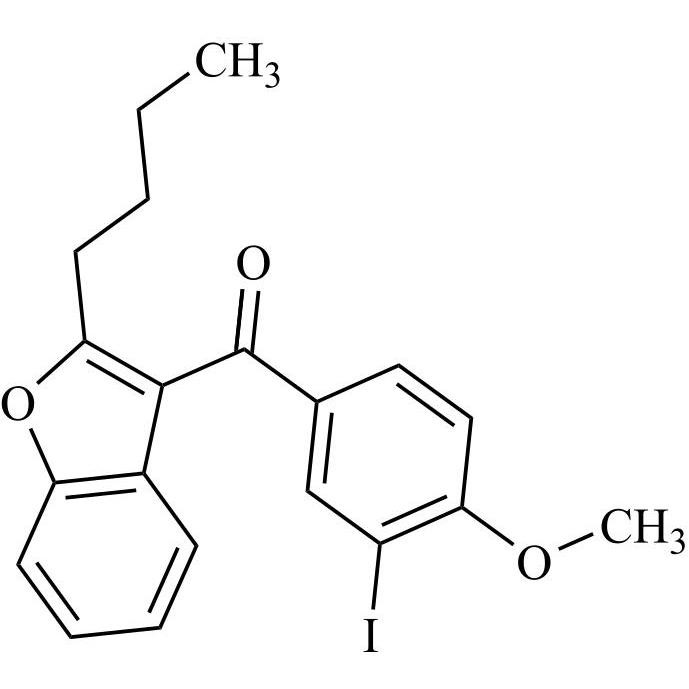
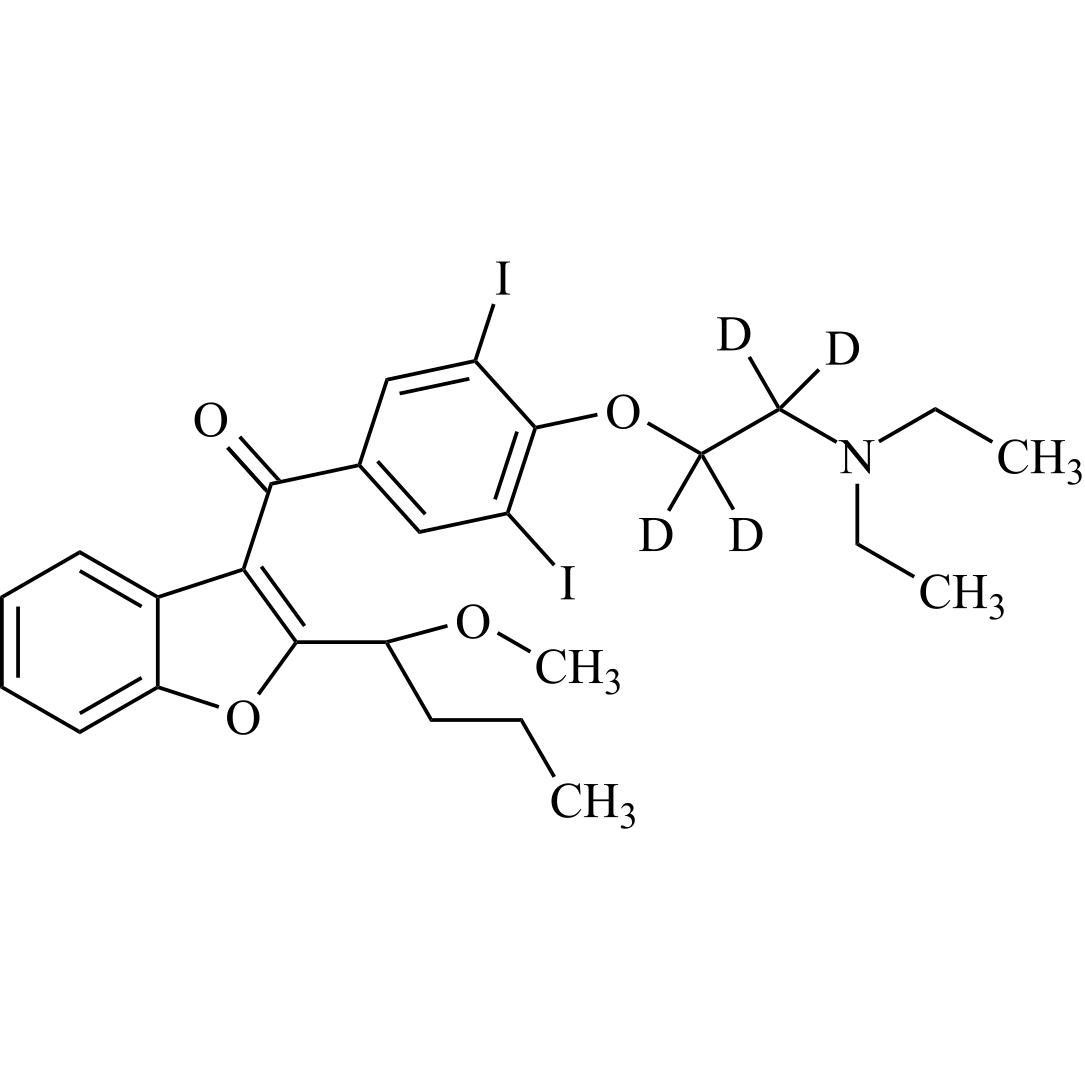
Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone
![Show details for Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone Picture of Des-O-[2-(diethylamino)ethyl]-1-methoxy Amiodarone](https://bc3c83a0874864a50753-7010fd7f2bdf81ab41ee28479491701e.ssl.cf2.rackcdn.com/media//catalog/AR-A06279.jpg?size=256)
M.F.
M.W. 576.17
CAT# AR-A06279
CAS# 1391054-75-3